Pitavastatin calcium intermediate preparation method
A technology for pitavastatin calcium and intermediates, applied in the field of preparation of pitavastatin calcium intermediates, can solve problems such as not easy to completely remove, dimethyl sulfoxide waste water, triphenoxyphos solid waste, etc., and achieve beneficial The protection of the ecological environment, the maximum application value, the effect of reducing solid waste and liquid waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
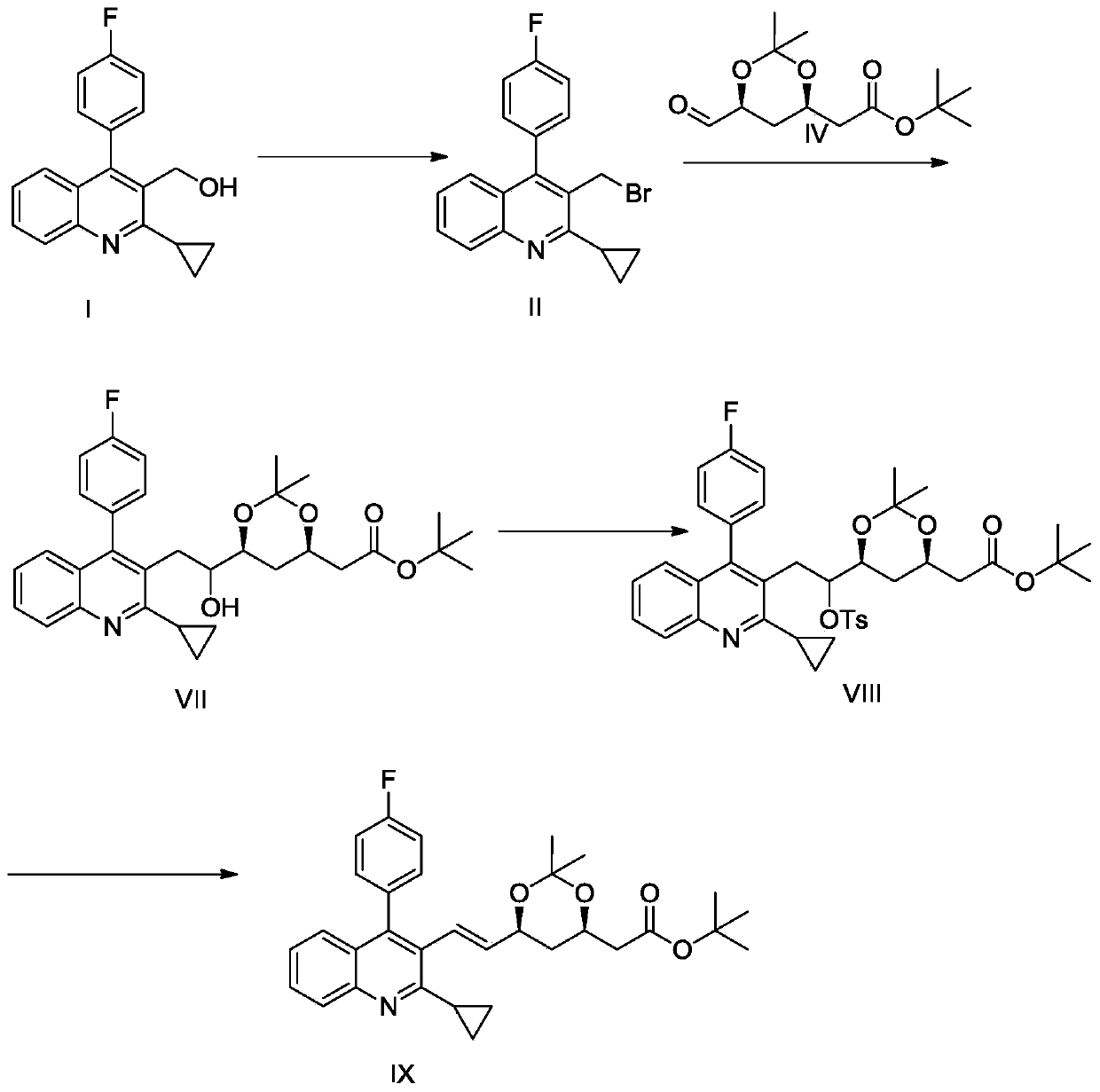
[0019] 2-((4R,6S)-6-((E)-2-(2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)ethenyl)-2,2-dimethyl Preparation of (1,3-dioxan-4-yl) tert-butyl acetate compound V:
[0020] Pump 700 kg of methylene chloride into a 2000-liter reactor, then add 100 kg of (2-cyclopropyl-4-(fluorophenyl)quinolin-3-yl)methanol, start stirring, and cool down to 0 to 5°C; Take 90 kg of phosphorus tribromide to the high level tank, start dropping, keep the reaction temperature at 0 to 5 °C during the dropping process, keep the temperature at 0 to 5 °C for one hour after the addition; add 100 kg of water to quench the reaction, keep the temperature at 0 to 5°C, stirred for 1 hour, added dropwise 15% sodium hydroxide aqueous solution to adjust the pH to 8, separated the dichloromethane layer, washed three times with water; concentrated most of the dichloromethane under reduced pressure, added 500 liters of tetrahydrofuran, and reduced pressure Concentrate 50 liters of tetrahydrofuran to obtain a tetrahydro...
Embodiment 2
[0022] 2-((4R,6S)-6-((E)-2-(2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)ethenyl)-2,2-dimethyl Preparation of (1,3-dioxan-4-yl) tert-butyl acetate compound V:
[0023] Add 200 milliliters of tetrahydrofuran into a 3-liter three-necked flask, then add 23 grams of active zinc powder, 0.5 grams of iodine, replace with nitrogen three times, heat to 50 to 60 ° C under stirring and keep for 30 minutes until the color of iodine disappears; 121 grams of 3-(methyl bromide Base)-2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline was dissolved in 450 milliliters of tetrahydrofuran, and then added dropwise to the reaction flask, and the rate of addition was controlled so that the reaction temperature was between 50 and 60°C. After completion of the heat preservation reaction for 2 hours, the temperature of the reaction system was lowered to 10 to 20°C, and 88 grams of 2-((4R,6S)-6-formyl-2,2-dimethyl-1,3-dioxane-4 - Base) tetrahydrofuran (250 ml) solution of tert-butyl acetate was added dropw...
Embodiment 3
[0025] 2-((4R,6S)-6-((E)-2-(2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)ethenyl)-2,2-dimethyl Preparation of (1,3-dioxan-4-yl) tert-butyl acetate compound V:
[0026]Add 200 milliliters of toluene to a 3-liter there-necked flask, then add 23 grams of active zinc powder, 0.5 gram of iodine, replace with nitrogen three times, heat to 60 to 70 ° C under stirring and keep for 30 minutes until the color of iodine disappears; 121 grams of 3-(methyl bromide Base)-2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline was dissolved in 600 milliliters of toluene, and then added dropwise to the reaction flask, and the rate of addition was controlled so that the reaction temperature was between 60 and 70°C. After completion of the heat preservation reaction for 2 hours, the temperature of the reaction system was lowered to 10 to 20°C, and 88 grams of 2-((4R,6S)-6-formyl-2,2-dimethyl-1,3-dioxane-4 - Base) toluene (400 ml) solution of tert-butyl acetate is added dropwise to the reaction flask, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com