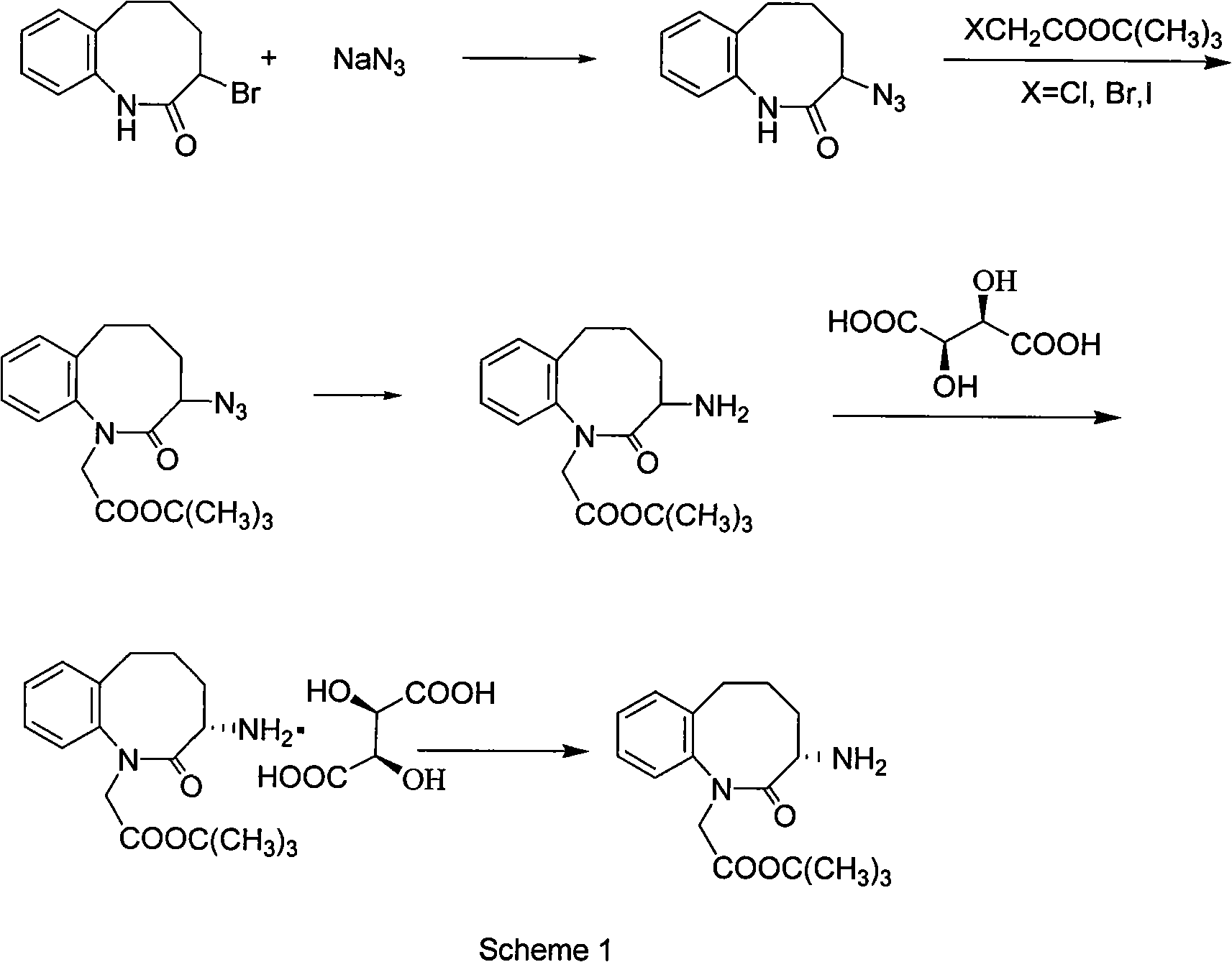
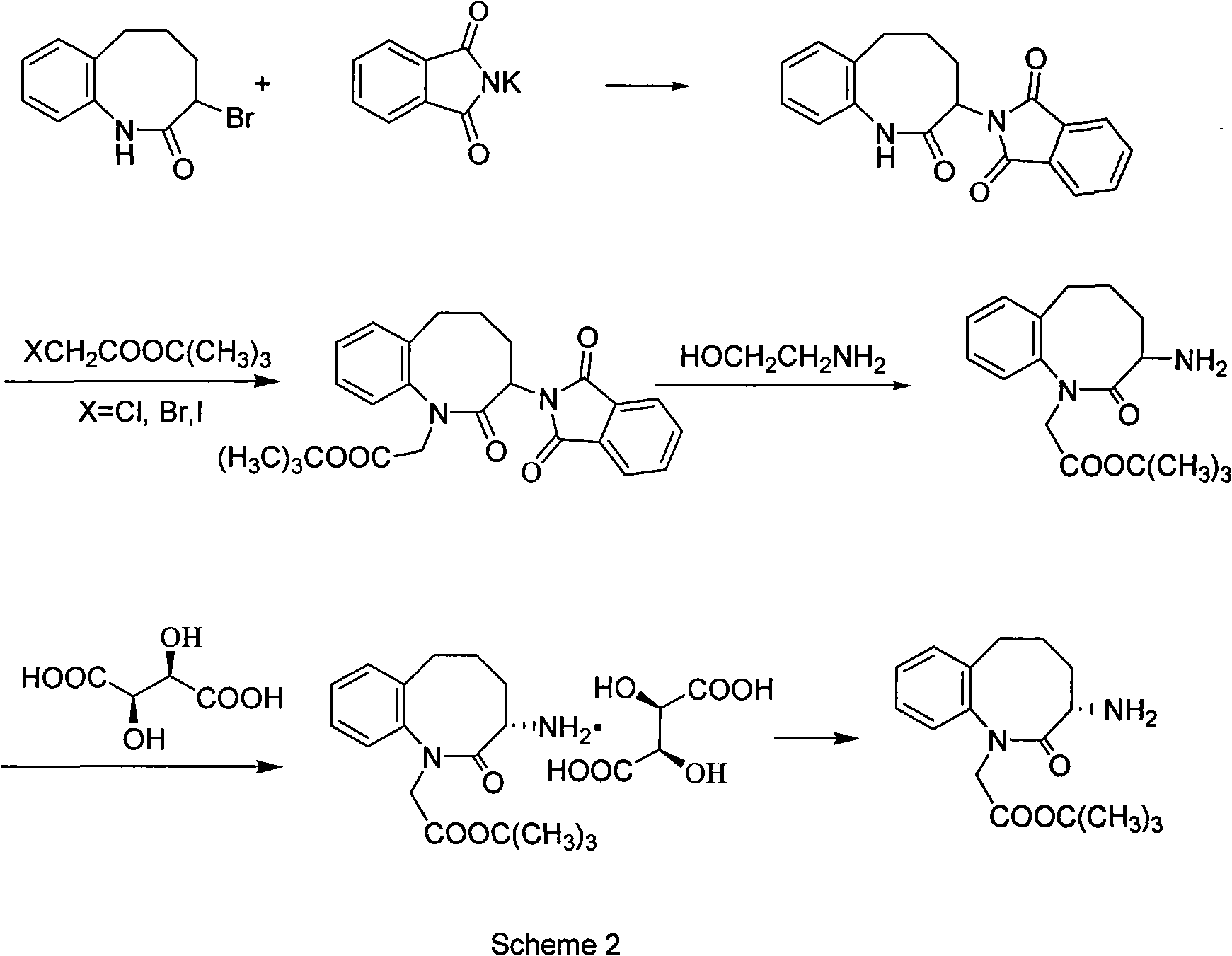
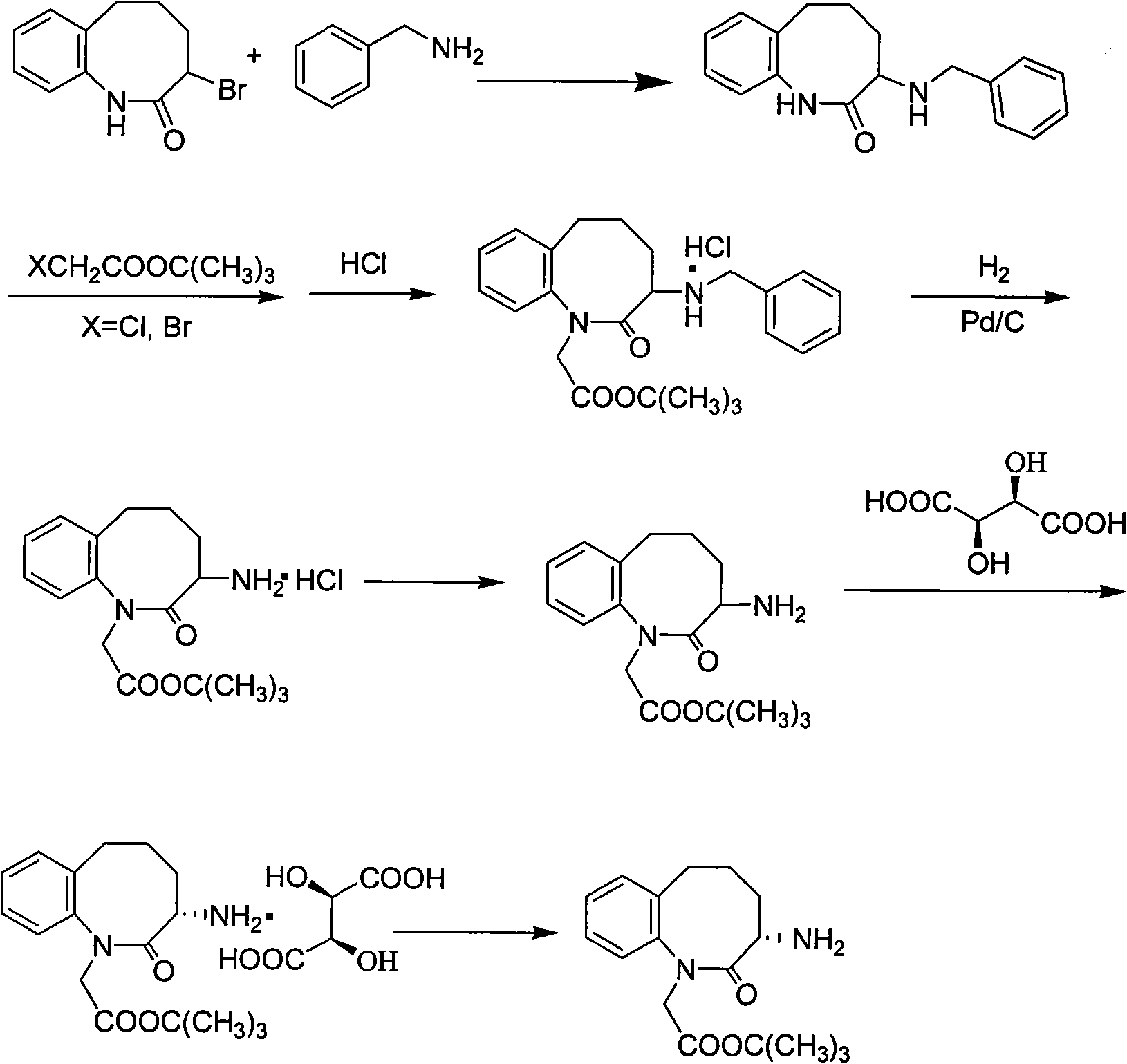
Method for preparing 3-(S)- amino-2,3,4,5-tetrahydro-2-oxy-1H-1-benzazepin-1-tert-butyl acetate
A technology of tert-butyl acetate and benzonitrogen, applied in the field of antihypertensive drugs, can solve the problems of high cost, unsatisfactory yield, large amount of solvent, etc., and achieve the effects of high cost, avoiding dangerous operation, and less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0025] first step:
[0026] Put 80g of 3-bromo-1,3,4,5-tetrahydro-2H-1-benzazepin-2-one, 40g of benzylamine, 40g of potassium carbonate, and 3g of tetrabutylammonium bromide into a 500ml three-necked bottle , Methanol 200ml, heat up and reflux reaction, TLC control, after the reaction is completed, cool down to 5-8°C, filter with suction, stir and wash the filter cake with 200ml of water for 30 minutes, filter with suction, and vacuum dry the filter cake at 70°C for 10 hours to obtain 82 g of 3-benzylamino-2,3,4,5-tetrahydro-2-oxo-1H-1-benzazepine (92.5% yield).
[0027] Step two:
[0028] Put 80g of 3-benzylamino-2,3,4,5-tetrahydro-2-oxo-1H-1-benzazepine into a 1000ml three-necked bottle, 600ml of benzene, 59g of tert-butyl bromoacetate, tetrabutyl Add 5g of ammonium bromide, add 1.4g of solid sodium hydroxide, heat up and reflux for 1 hour, control by TLC, after the reaction is completed, cool down to about 25°C, filter with suction, discard the filter cake, distill the fi...
example 2
[0038] first step:
[0039] Put 80g of 3-bromo-1,3,4,5-tetrahydro-2H-1-benzazepin-2-one, 40g of benzylamine, 40g of potassium carbonate, and 3g of tetrabutylammonium bromide into a 500ml three-necked bottle 1. Isopropanol 200ml, heat up and reflux reaction, TLC control, after the reaction is completed, cool down to 5-8°C, filter with suction, stir and wash the filter cake with 200ml of water for 30 minutes, filter with suction, and vacuum dry the filter cake at 70°C for 10 hours , to obtain 81.3 g of 3-benzylamino-2,3,4,5-tetrahydro-2-oxo-1H-1-benzazepine (yield 92.9%).
[0040] Step two:
[0041] 80g of 3-benzylamino-2,3,4,5-tetrahydro-2-oxo-1H-1-benzazepine, 630ml of toluene, 53g of tert-butyl chloroacetate, 53g of tetrabutyl Add 5g of ammonium bromide, add 1.9g of solid potassium hydroxide, heat up and reflux for 1 hour, control by TLC, after the reaction is completed, cool down to about 25°C, filter with suction, discard the filter cake, and distill the filtrate under re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com