Reverse sex pheromone for prevention and control of plutella xylostella l.
A technology of anti-sex pheromone and diamondback moth, which is applied in the fields of chemicals for biological control, pest control, application, etc., can solve the problems of not yet found the sex pheromone of Plutella xylostella, strong interference of anti-sex pheromone, etc. Environmentally friendly, reducing the number of mating, reducing the effect of harm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
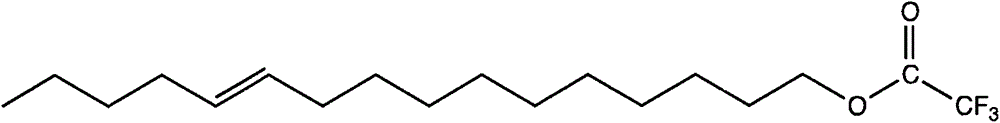
[0015] The preparation of embodiment 1 cis-11-hexadecenol trifluoromethyl ketone
[0016] The molecular formula of cis-11-hexadecenol trifluoromethyl ketone is as follows:
[0017]
[0018] Its preparation method is as follows:
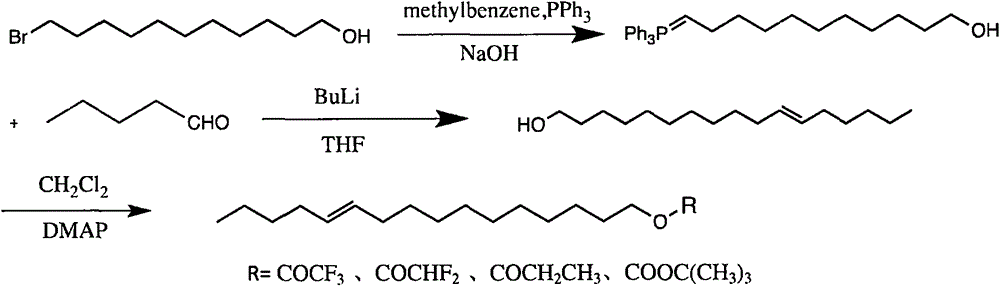
[0019] 1) Preparation of phosphorus ylides
[0020] Dissolve 5g (19.9mmol) of 11-bromo-1-undecanol in 40ml of acetonitrile, add 6.27g (23.9mmol) of triphenylphosphine, reflux at 81-82°C for 72h, evaporate the solvent to dryness, and obtain a white paste Add 1 mol / L NaOH aqueous solution, stir at room temperature, and filter to obtain 6.80 g (15.73 mmol) of white solid, with a yield of 79%. 1HNMR (300MHz, internal standard TMS, solvent CDCl 3 )δ1.26-1.29(m, 12H), 1.43-1.53(m, 6H), 3.50(m, 2H), 7.30-7.51(m, 15H), 11.0(br, 1H).
[0021] 2) Synthesis of cis-11-hexadecen-1-ol
[0022] Under the protection of high-purity nitrogen, dissolve the phosphorus ylide prepared in 1 in anhydrous 30ml THF, cool down to -78°C, slowly add 1g (15.73mmol) butylli...
Embodiment 2
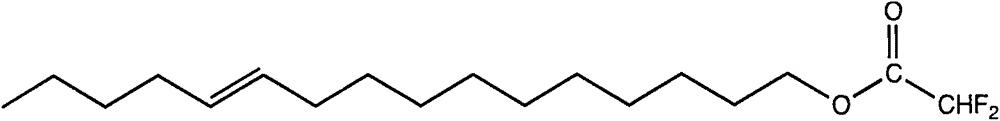
[0024] Embodiment 2 The preparation of cis-11-hexadecenol difluoromethyl ketone
[0025] The molecular formula of cis-11-hexadecenol difluoromethyl ketone is as follows:
[0026]
[0027] Its preparation method is as follows:
[0028] 1) Preparation of phosphorus ylides
[0029] Dissolve 5g (19.9mmol) of 11-bromo-1-undecanol in 40ml of acetonitrile, add 6.27g (23.9mmol) of triphenylphosphine, reflux at 81-82°C for 72h, evaporate the solvent to dryness, and obtain a white paste Add 1 mol / L NaOH aqueous solution, stir at room temperature, and filter to obtain 6.80 g (15.73 mmol) of white solid, with a yield of 79%. 1 HNMR (300MHz, internal standard TMS, solvent CDCl 3 )δ1.26-1.29(m, 12H), 1.43-1.53(m, 6H), 3.50(m, 2H), 7.30-7.51(m, 15H), 11.0(br, 1H).
[0030] 2) Synthesis of cis-11-hexadecen-1-ol
[0031] Under the protection of high-purity nitrogen, dissolve the phosphorus ylide prepared in 1 in anhydrous 30ml THF, cool down to -78°C, slowly add 1g (15.73mmol) butyllit...
Embodiment 3
[0033] The preparation of embodiment 3 cis-11-hexadecene-1-propionate
[0034] The molecular formula for cis-11-hexadecene-1-propionate is shown below:
[0035]
[0036] Its preparation method is as follows:
[0037] 1) Preparation of phosphorus ylides
[0038] Dissolve 5g (19.9mmol) of 11-bromo-1-undecanol in 40ml of acetonitrile, add 6.27g (23.9mmol) of triphenylphosphine, reflux at 81-82°C for 72h, evaporate the solvent to dryness, and obtain a white paste Add 1 mol / L NaOH aqueous solution, stir at room temperature, and filter to obtain 6.80 g (15.73 mmol) of white solid, with a yield of 79%. 1 HNMR (300MHz, internal standard TMS, solvent CDCl 3 )δ1.26-1.29(m, 12H), 1.43-1.53(m, 6H), 3.50(m, 2H), 7.30-7.51(m, 15H), 11.0(br, 1H).
[0039] 2) Synthesis of cis-11-hexadecen-1-ol
[0040]Under the protection of high-purity nitrogen, dissolve the phosphorus ylide prepared in 1 in anhydrous 30ml THF, cool down to -78°C, slowly add 1g (15.73mmol) butyllithium in batches, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com