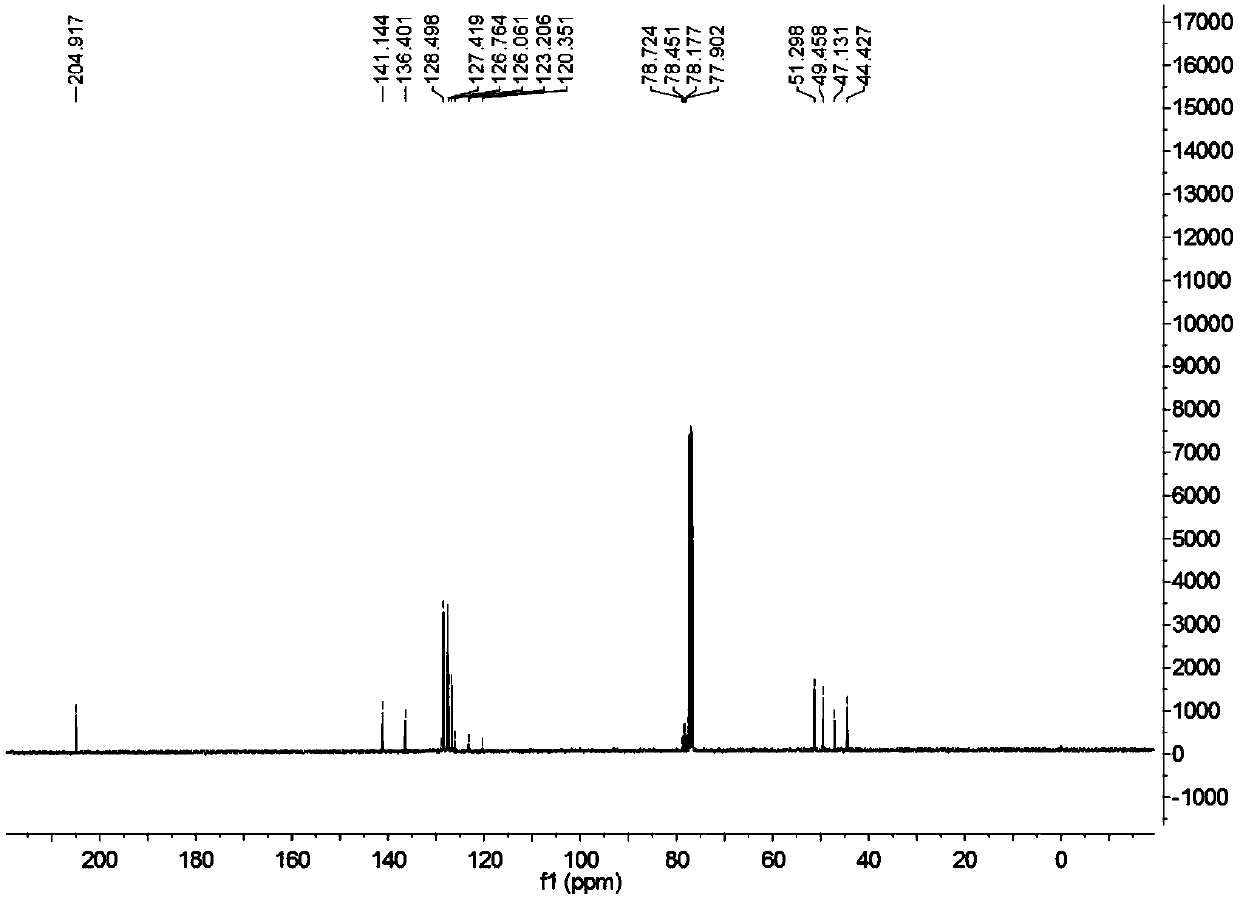
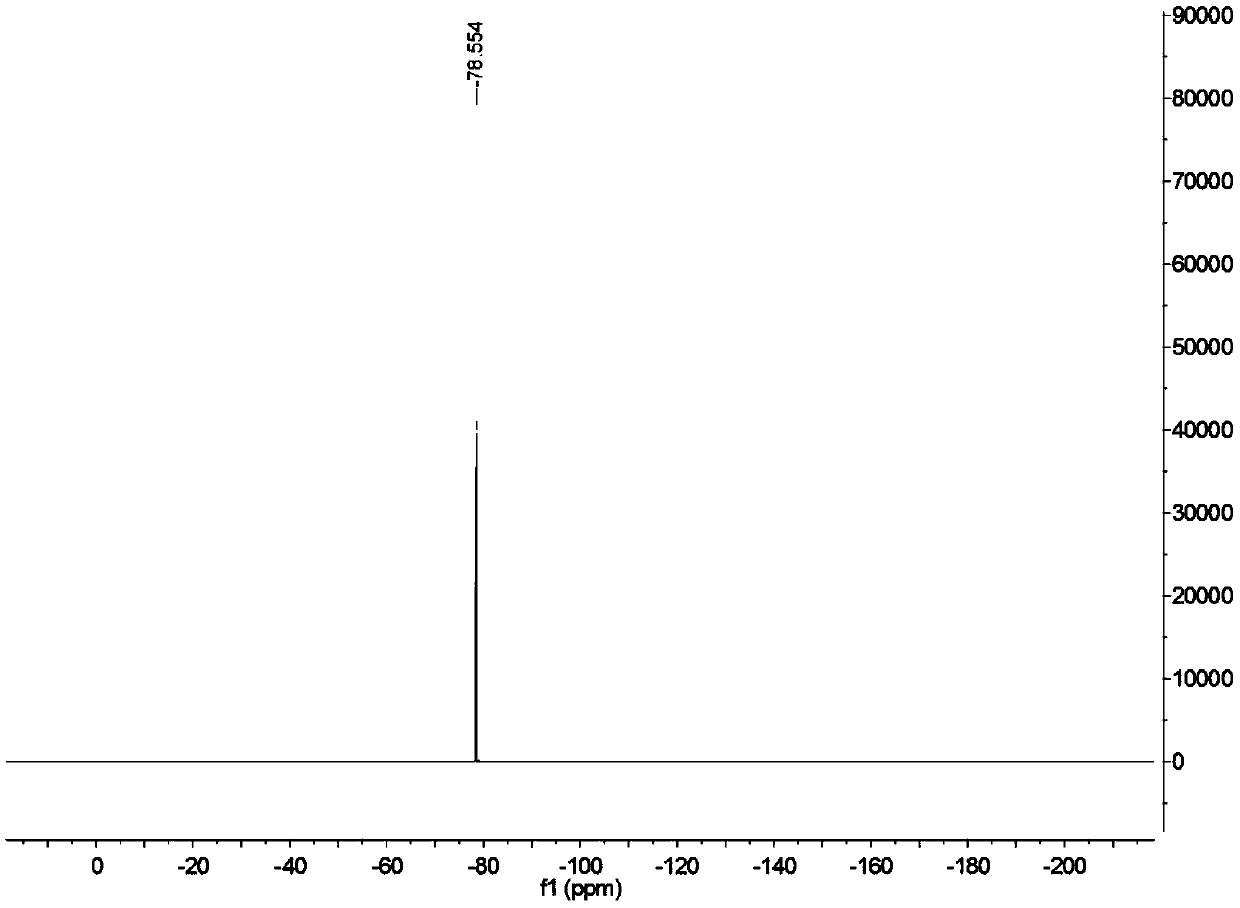
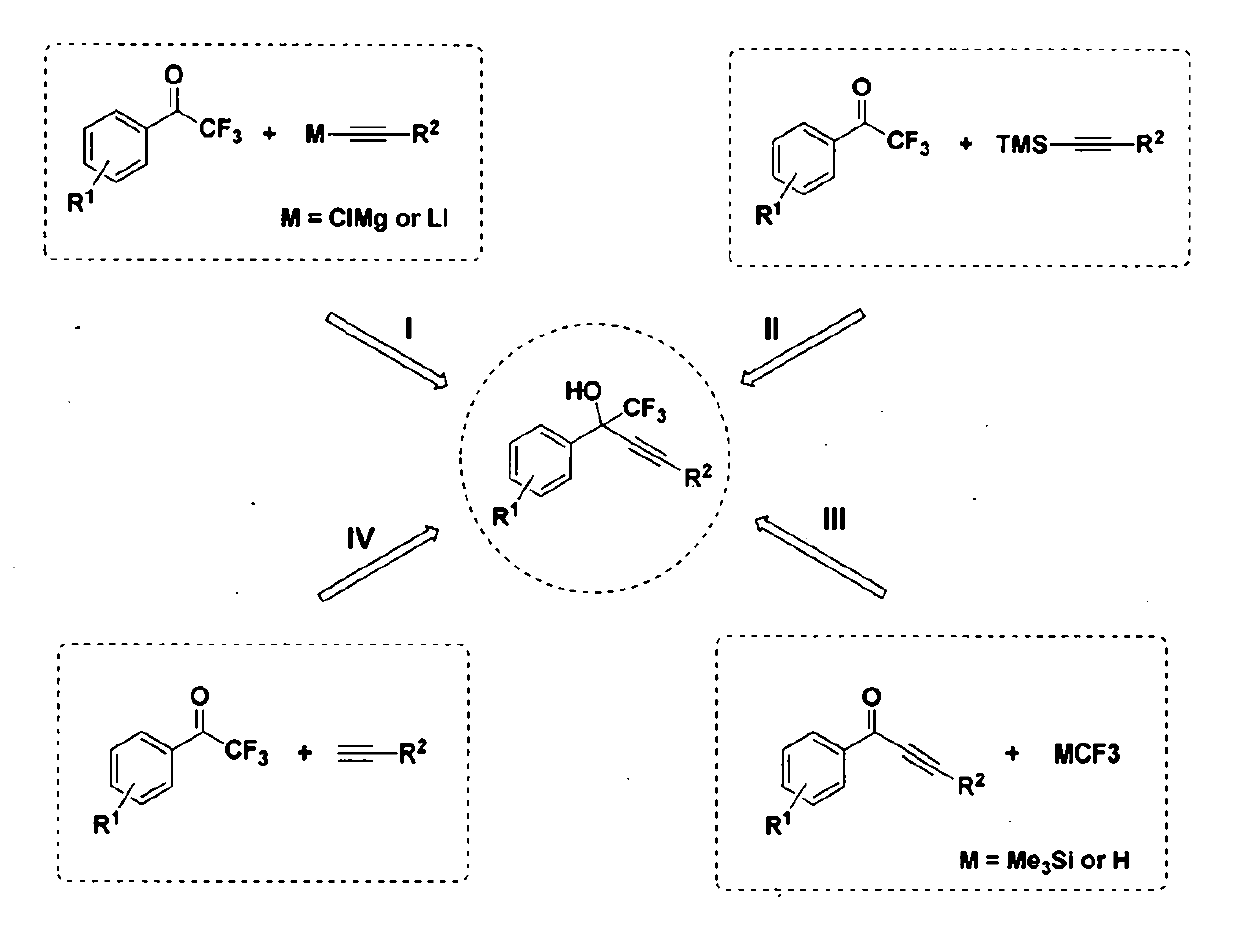
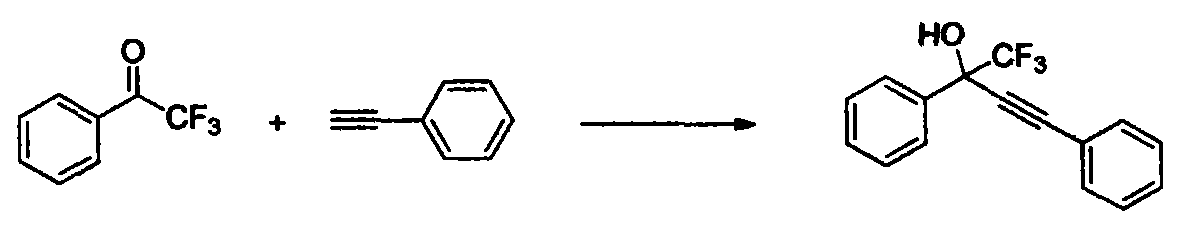
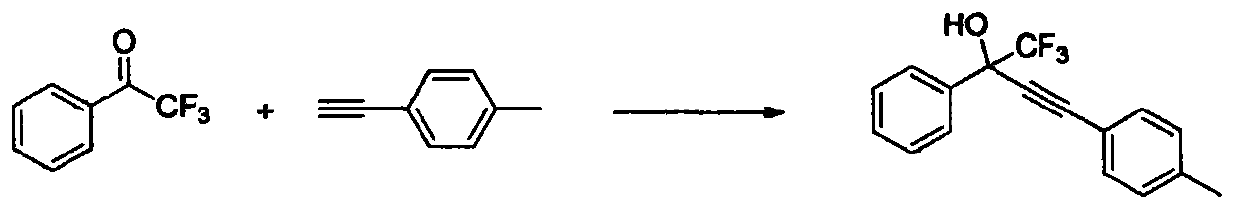
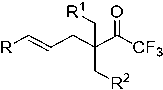
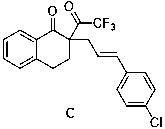
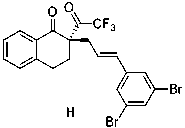
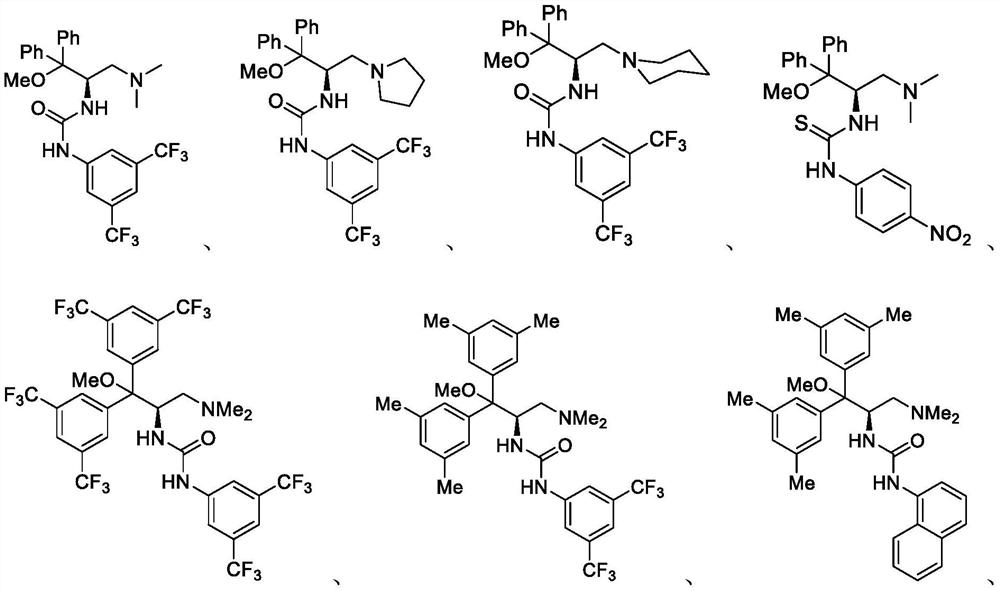
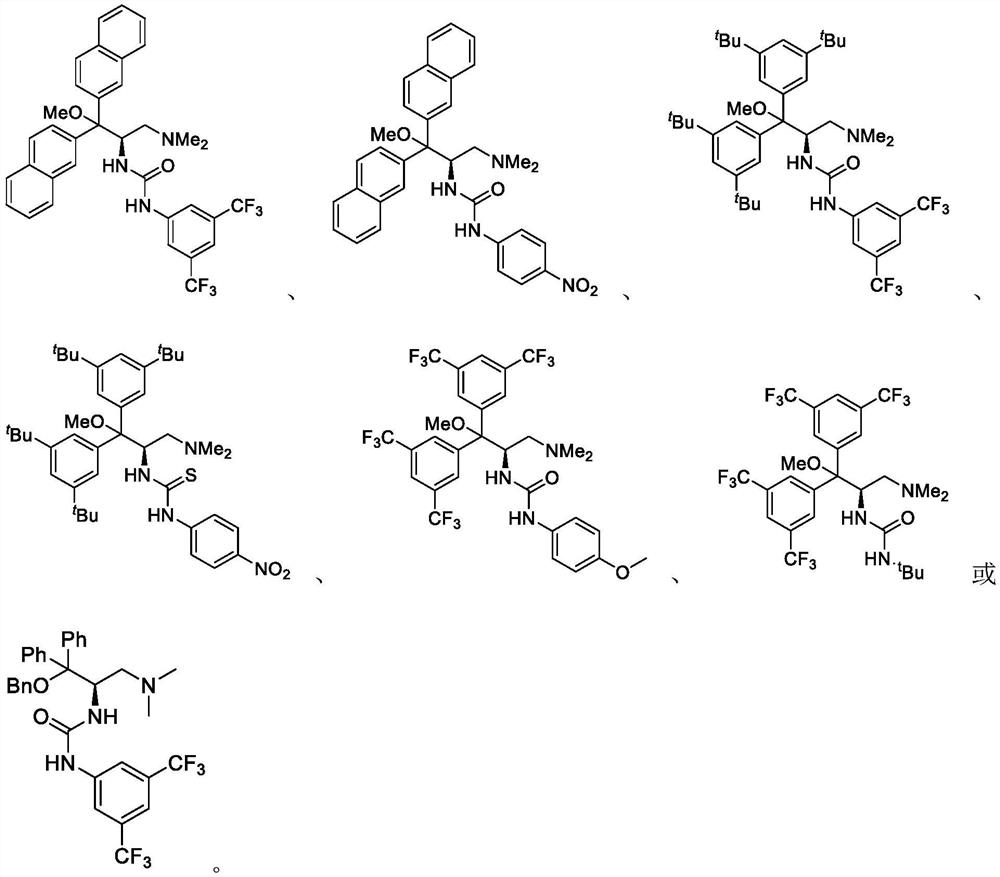
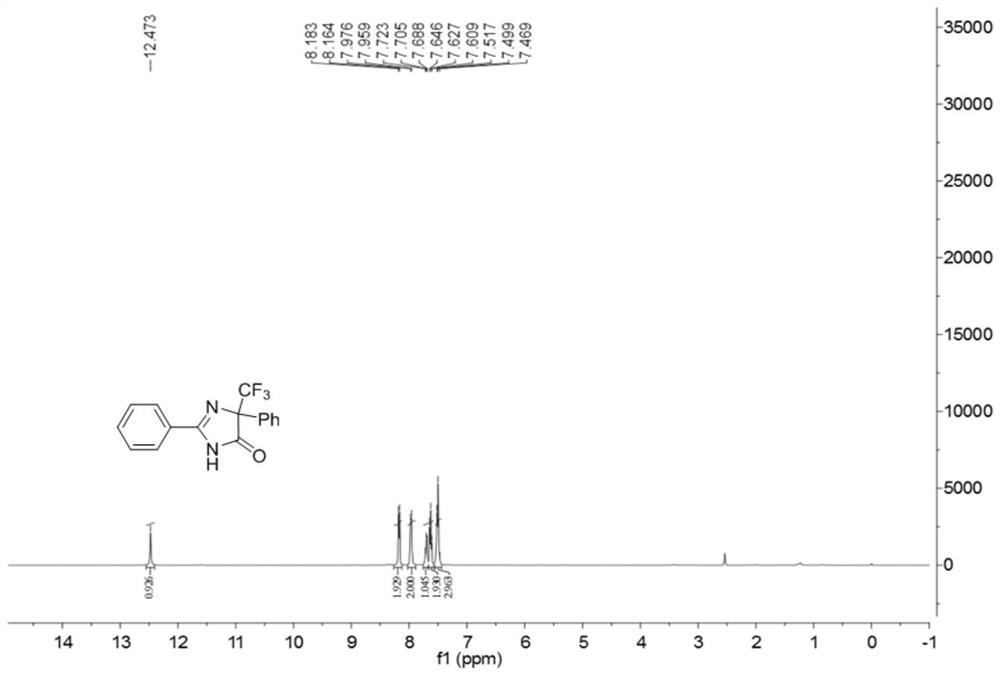
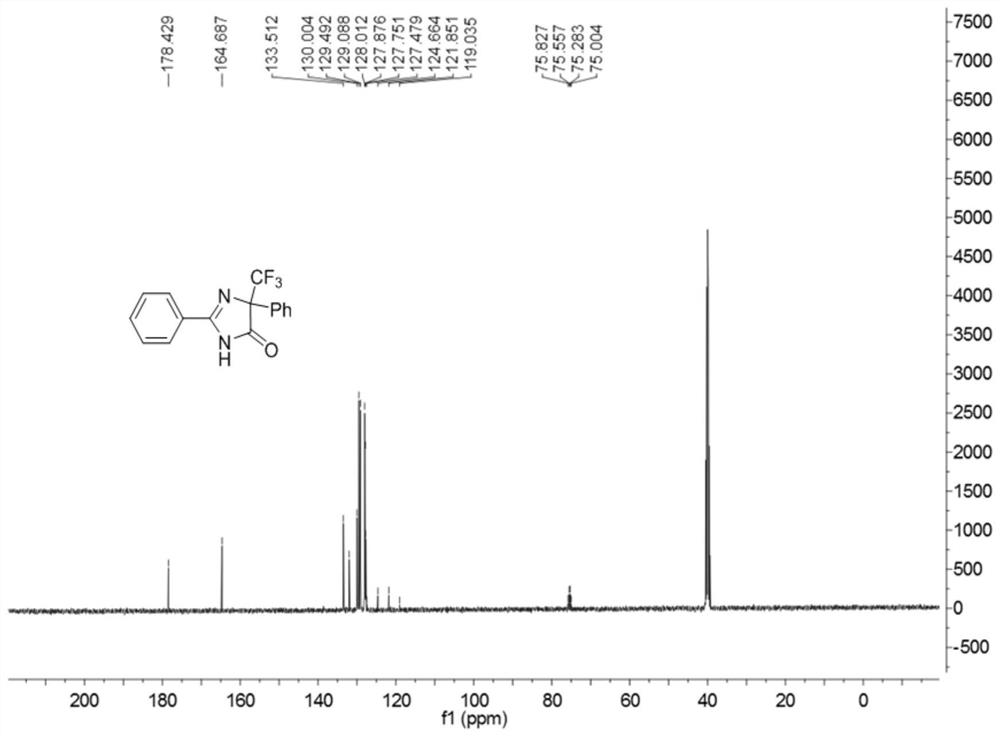
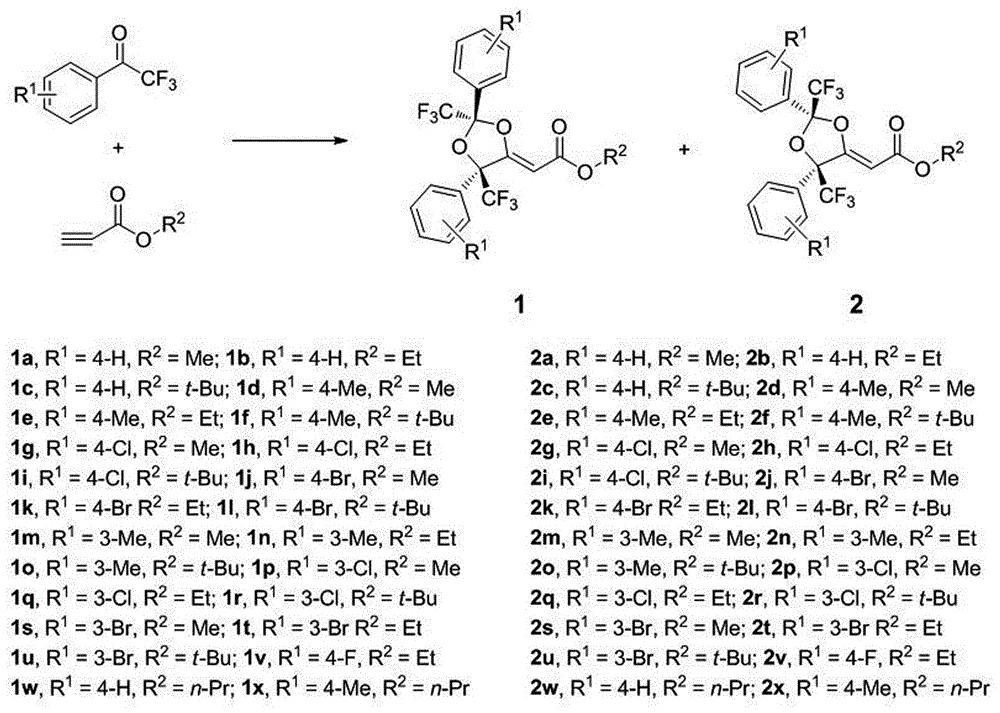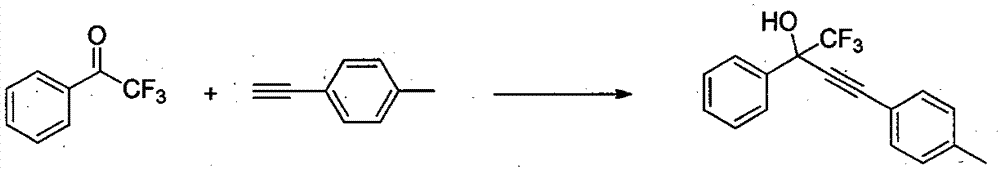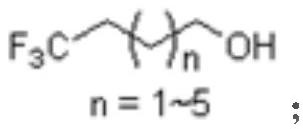Patents
Literature
37 results about "Trifluoromethyl ketone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Arachidonyl trifluoromethyl ketone (ATK) is an analog of arachidonic acid. that inhibits some isoforms of the enzyme phospholipase A2. Specifically it inhibits the 85 kDa cystolic PLA2 (cPLA2).. It has been studied as a neuroprotective agent after spinal cord injury, and in animal models of multiple sclerosis.. See also. Spinal stenosis
Reverse sex pheromone for prevention and control of plutella xylostella l.
InactiveCN105123718AInhibition orientationInterfering with matingBiocidePest attractantsPropionateMammal
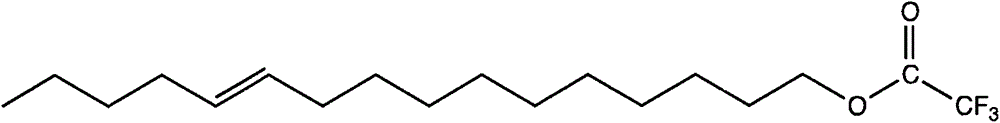
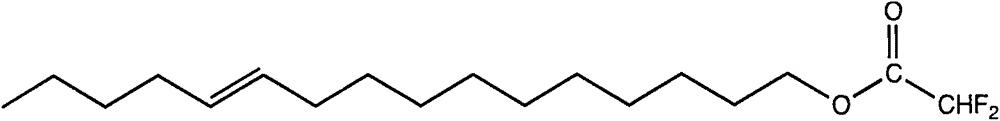
The invention discloses a reverse sex pheromone for interfering plutella xylostella l. mating, and belongs to the biological prevention and control field. The reverse sex pheromone is one or more of cis-11-cetylenol trifluoromethyl ketone, cis-11-cetylenol difluoromethyl ketone, cis-11-hexadecene-1-propionate and cis-11-hexadecene-1-tertiary butyl acetate. The reverse sex pheromone can significantly inhibit orientation of plutella xylostella l. male adults on female sex pheromone, thereby interfering plutella xylostella l. mating, and reducing the field insect population number. The reverse sex pheromone has the advantages of high efficiency, multiple ecological effects, simple preparation, environmental friendliness, safety to mammals and the like, can reduce the field male and female meeting probability and mating frequency, so as to relieve harm of plutella xylostella l. on cruciferous plants.
Owner:INST OF PLANT PROTECTION CHINESE ACAD OF AGRI SCI
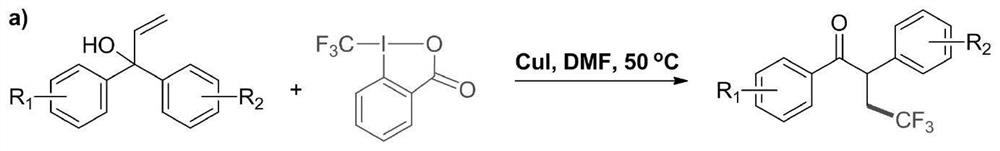
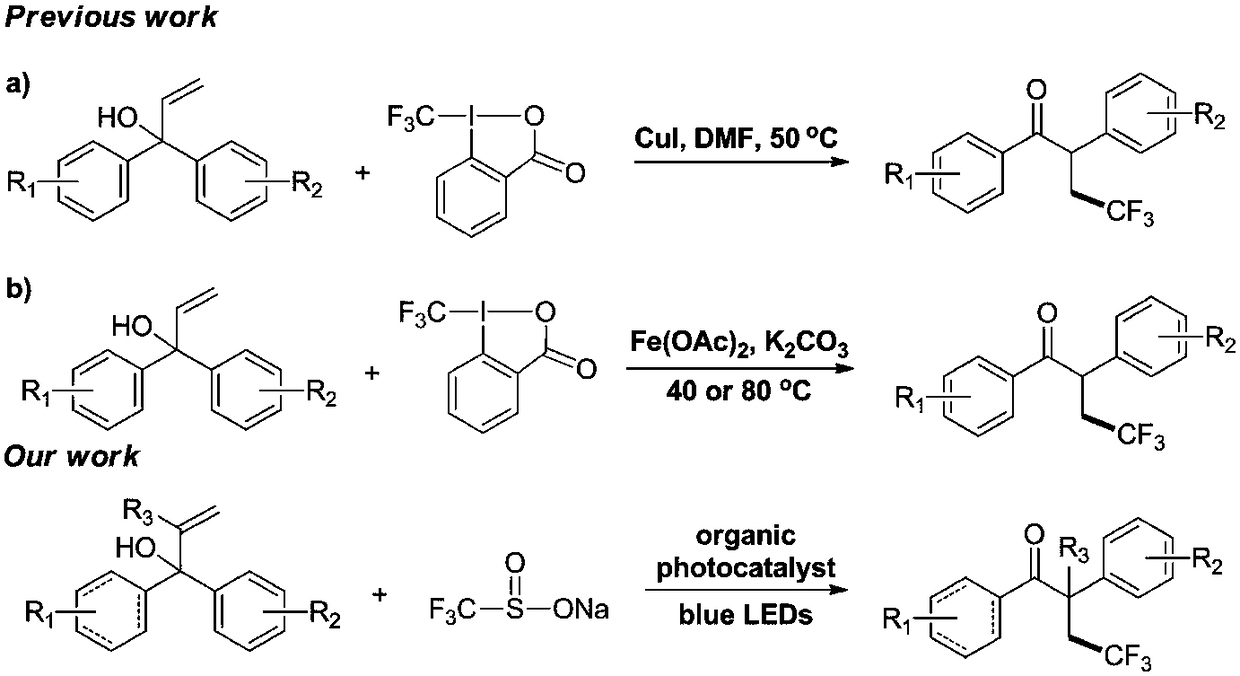
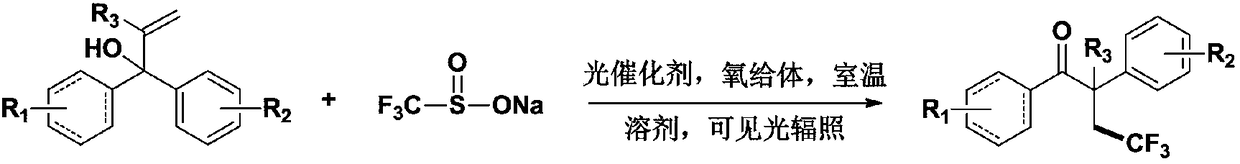
Method for preparing alpha-aryl-beta-trifluoromethyl ketone compound through visible light catalysis
ActiveCN108774121AImprove toleranceLow priceOrganic compound preparationCarbonyl compound preparationOrganic synthesisOxygen donor
The invention discloses a method for preparing an alpha-aryl-beta-trifluoromethyl ketone compound through visible light catalysis, which is characterized in that in an oxygen atmosphere, an alpha-aryl-beta-trifluoromethyl ketone compound is obtained by synthesis of alpha-mono-aryl allyl alcohol compound and sodium trifluoromethanesulfonate as materials under the action of the synergistic effect ofa photocatalyst, visible light, a solvent and an oxygen donor. According to the invention, a new method for preparing the alpha-aryl-beta-trifluoromethyl ketone compound is disclosed by performing visible light photocatalysis reaction on a series of alpha-mono-aryl allyl alcohol material and Langlois reagent under a mild reaction condition. The catalyst system has good tolerance for synthesis ofvarious useful functional groups. According to the method, no strong oxidant or transition metal catalyst is needed, and sodium trifluoromethanesulfonate which is easy to obtain and relatively low incost is taken as a starting material, so that further the application of the synthetic method in organic synthesis is added.
Owner:MINNAN NORMAL UNIV
Preparation method of trifluoromethyl ketone
InactiveCN103224447AIncrease added valueImprove versatilityOrganic compound preparationCarbonyl compound preparationTrifluoroacetic acidOrganic layer
The invention provides a preparation method of trifluoromethyl ketone. The preparation method of the trifluoromethyl ketone comprises the following steps: (1) after being mixed, magnesium metal, solvent, halide and sodium trifluoroacetate are reacted for 0.5-2.5 hours at the temperature of 30-65 DEG C, and then are reacted for 1-3 hours at the rising temperature of 30-70 DEG C, the molar ratio of the halide and the magnesium metal is 1-1.2:1, the molar ratio of the solvent and the magnesium metal is 2-8:1, the molar ratio of the magnesium metal and the sodium trifluoroacetate is 1-1.5:1, and after the reaction finishes, obtained reaction liquid is cooled; (2) the reaction liquid is added to 2-4 mol / L of mineral acid and is acidized, an organic layer is collected, the organic layer is rectified and purified, and then the trifluoromethyl ketone is obtained. The trifluoromethyl ketone has the advantages of being low in raw material cost, low in production cost, easily purified and low in production equipment requirements, wherein the raw materials of the trifluoromethyl ketone are easy to get.
Owner:JUHUA GROUP TECH CENT
Trifluoroacetyl substituted hydrazone derivative and synthesis method thereof
ActiveCN109232310AImprove adaptabilityWide adaptabilityOrganic compound preparationHydrazone preparationSynthesis methodsOrganic synthesis
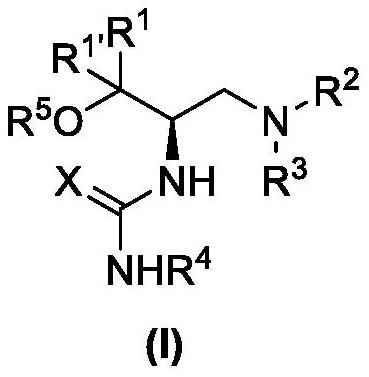
The invention belongs to the technical field of organic synthesis and discloses a trifluoroacetyl substituted hydrazone derivative and a synthesis method thereof. The trifluoroacetyl substituted hydrazone derivative has a structural formula shown as a formula (I); the synthesis method of the trifluoroacetyl substituted hydrazone derivative comprises the following steps: adding diazotate, trifluoromethyl ketone, alkali and a solvent into a reactor; stirring and reacting at 0 to 70 DEG C for 0.05 to 24h; after reaction is finished, cooling to room temperature; adding water and an organic solventand extracting a reaction solution; evaporating to remove the solvent, so as to obtain a crude product; and carrying out column chromatography purification to obtain the trifluoroacetyl substituted hydrazone derivative. The synthesis method provided by the invention does not utilizes a catalyst and does not need a ligand, and all raw materials have no toxicity and are cheap and easy to obtain; the reaction has good adaptability on functional groups, wide substrate adaptability and high product yield; and the synthesis method is simple and safe to operate and has moderate reaction conditions and a good industrial application prospect. The formula (I) is shown in the description.
Owner:SOUTH CHINA UNIV OF TECH
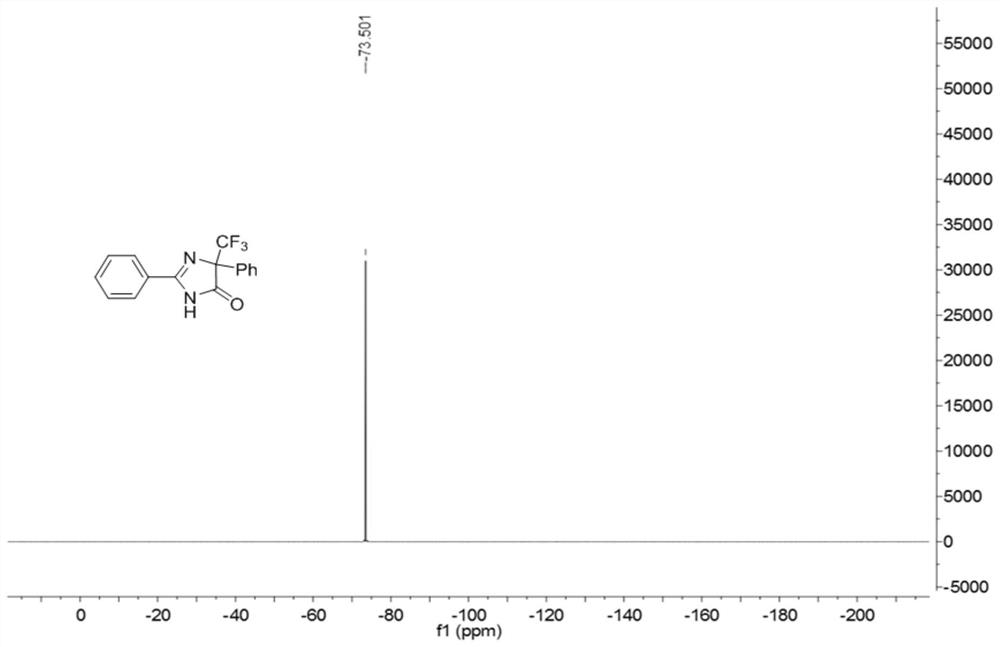
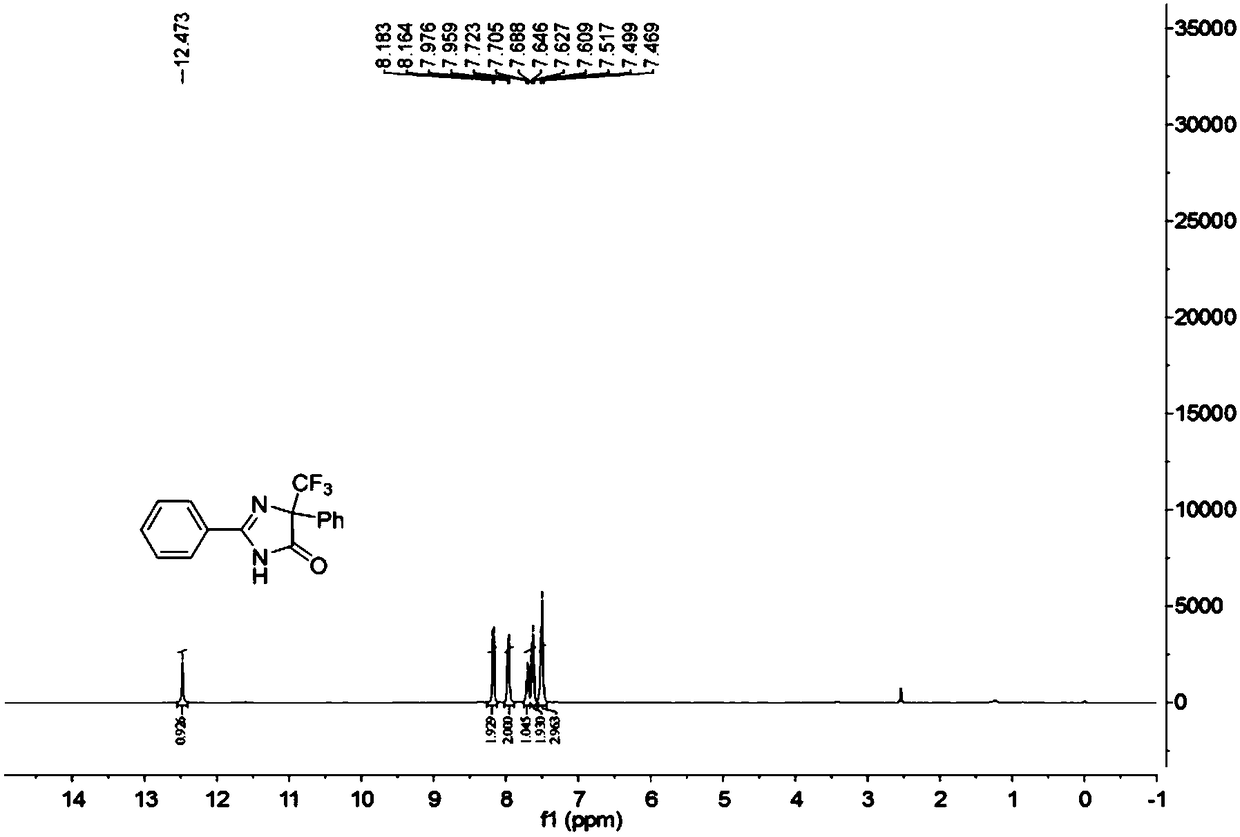
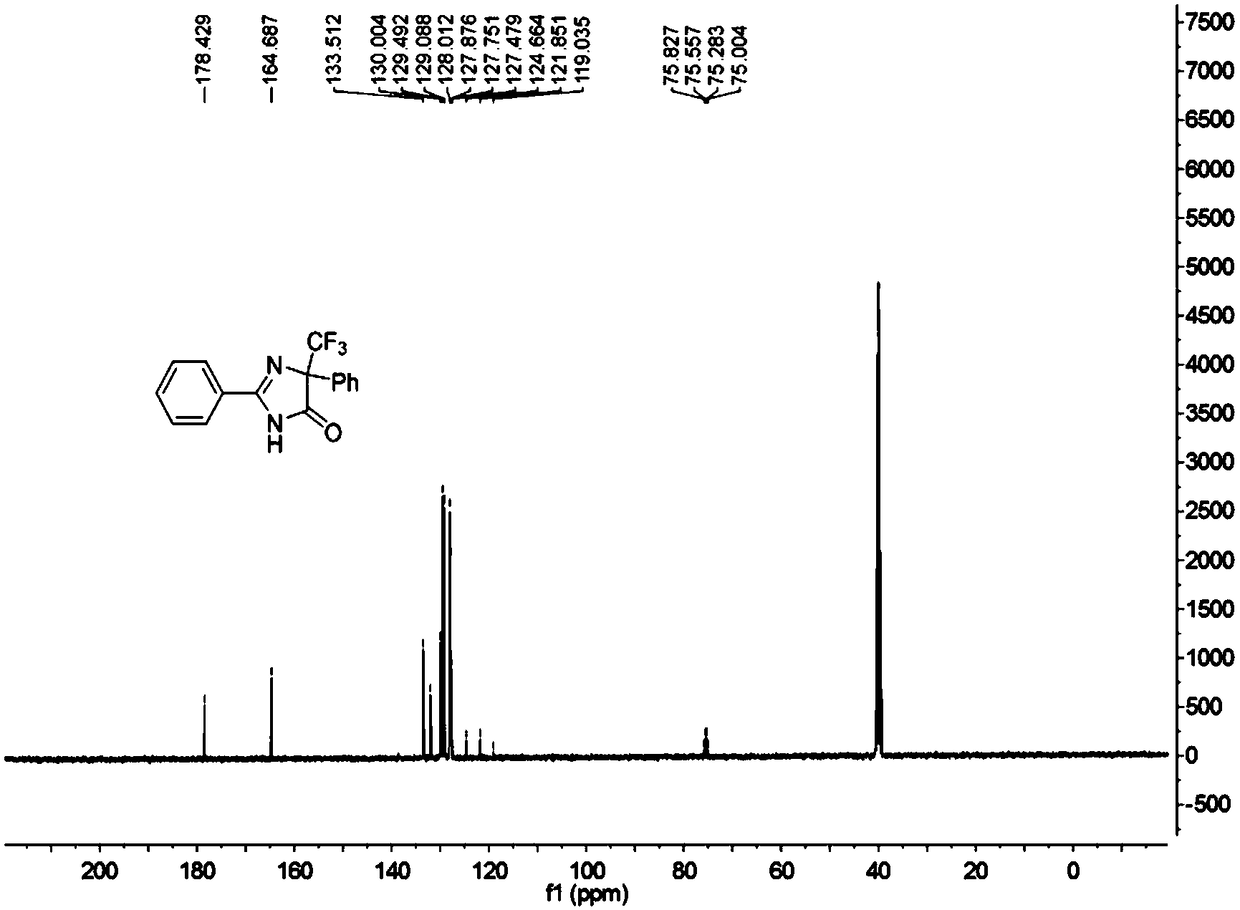
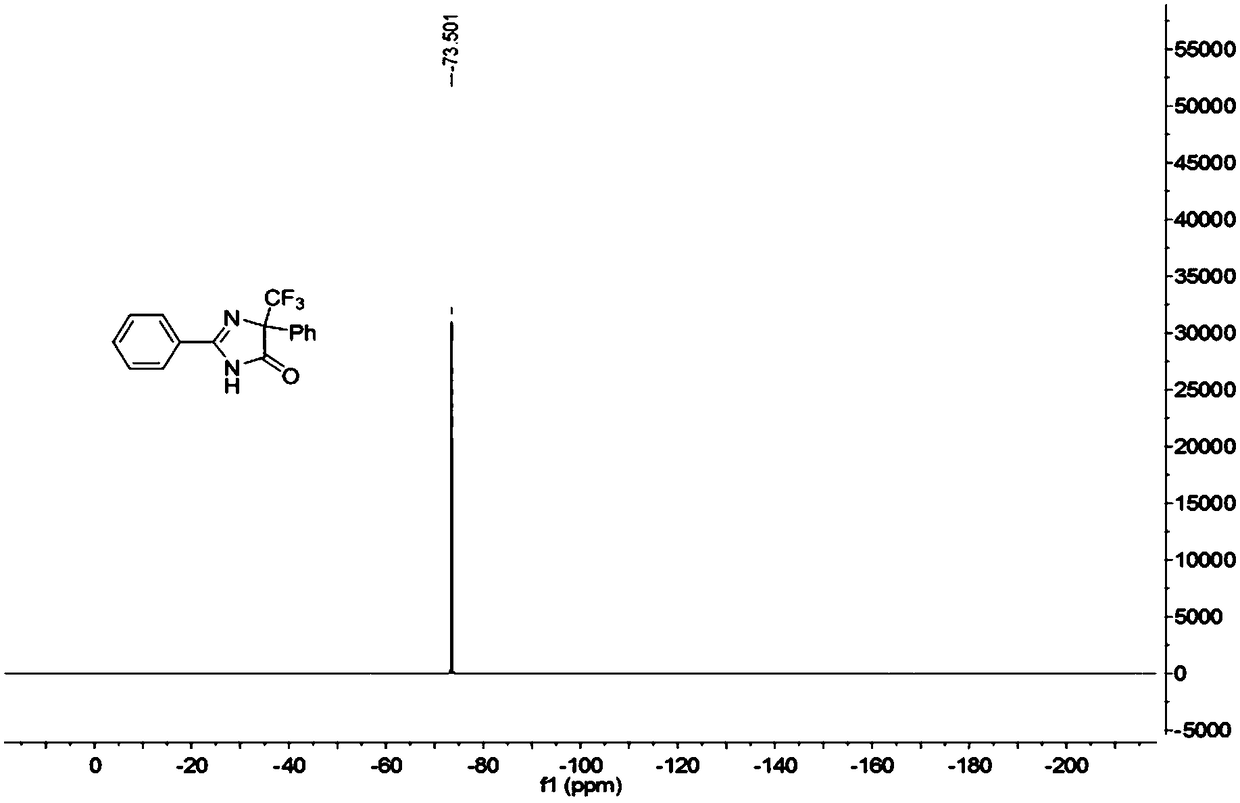
5-trifluoromethyl-4H-imidazoline-4-ketone derivative and synthetic method
ActiveCN108976170AImprove adaptabilityWide adaptabilityOrganic chemistryChemical synthesisOrganic solvent
The invention belongs to the technical field of medicine chemical synthesis, and discloses a 5-trifluoromethyl-4H-imidazoline-4-ketone derivative as well as a synthetic method. The synthetic method comprises the following steps: in an alkali and an organic solvent, enabling an amidine salt to react with trifluoromethyl ketone compound, and performing the subsequent treatment, thus obtaining the 5-trifluoromethyl-4H-imidazoline-4-ketone derivative. The structure of the 5-trifluoromethyl-4H-imidazoline-4-ketone derivative is as shown in formula I. By adopting the method of the invention, the useof a transitional metal catalyst can be avoided, and the used raw material is nontoxic, cheap and easy to obtain; and the reaction is good in adaptability to functional groups, wide in adaptability to the substrate, high in product yield, capable of realizing the mass production in a gram scale, and favorable for the industrial production, and the prepared product has wide use in the fields suchas pesticides, medicine, materials and the like. (Shown in the description).
Owner:SOUTH CHINA UNIV OF TECH
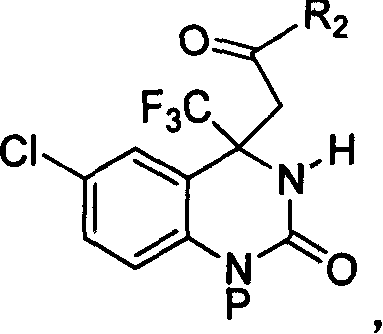
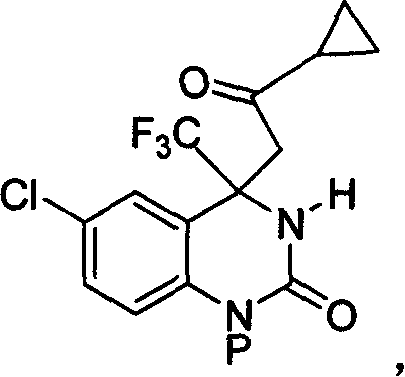
4,4-disubstituted-3,4-dihydro-2(1H)- quinolones and synthesis process and use thereof
InactiveCN1827605AHigh optical activityMild reaction conditionsOrganic active ingredientsOrganic chemistryQuinoloneFunction group
The invention relates to a 4, 4-disubstitutional-3,4-dihydro-2(one hydro)-quinaldinicketones compound and its method for synthesizing and its application. The said compound can be a compound with its constitutional formula on tha right, or its stereoisomer, its stereoisomeric mixture or a salt which can be accepted in the pharmacy. The method for synthesizing chiral pure isomer is carried out mainly by the asymmetric additive reaction between substrates of methyl ketone and trifluoromethylketone imine. Additionally, we can construct the most critical trifluoromethyl quaternary carbon chiral center of the target compound DPC083 easily and efficiently. Afterwards, the synthesis of the target compound DPC083 is realized by the transformation of the function group.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
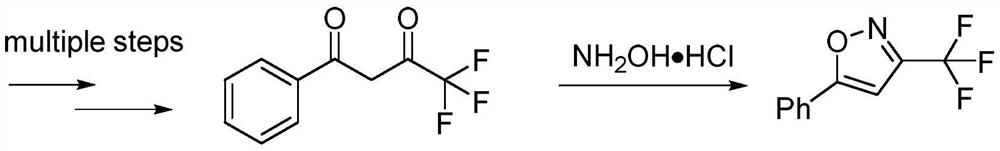
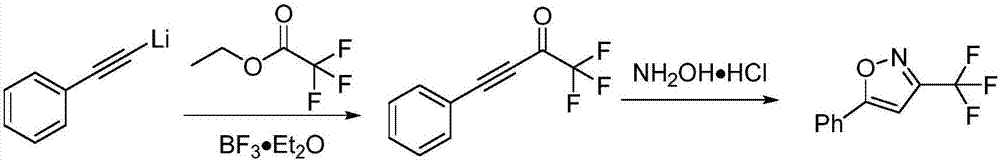
Method for preparing 3-trifluoromethylisooxazole compound by one-pot
ActiveCN107963996AEasy to operateMild conditionsGroup 4/14 element organic compoundsCopperCoupling reaction
The invention discloses a novel method for preparing a 3-trifluoromethylisooxazole compound by one-pot. According to the method, trifluoroethylamine which can be purchased in markets is prepared intofluorinated diazomethane, and then the fluorinated diazomethane and an alkynes compound are in coupled reaction under catalysis of low-price copper. The method is simple to operate, reaction conditions are mild, the cost is low, by-products are fewer, the yield is high, tolerance of a functional group is high, and reaction can be amplified. Meanwhile, much deeper mechanism study is carried out, and a mechanism that a trifluoromethyl ketoximes compound intermediate may be produced in the reaction is proposed.
Owner:JIANGXI NORMAL UNIV
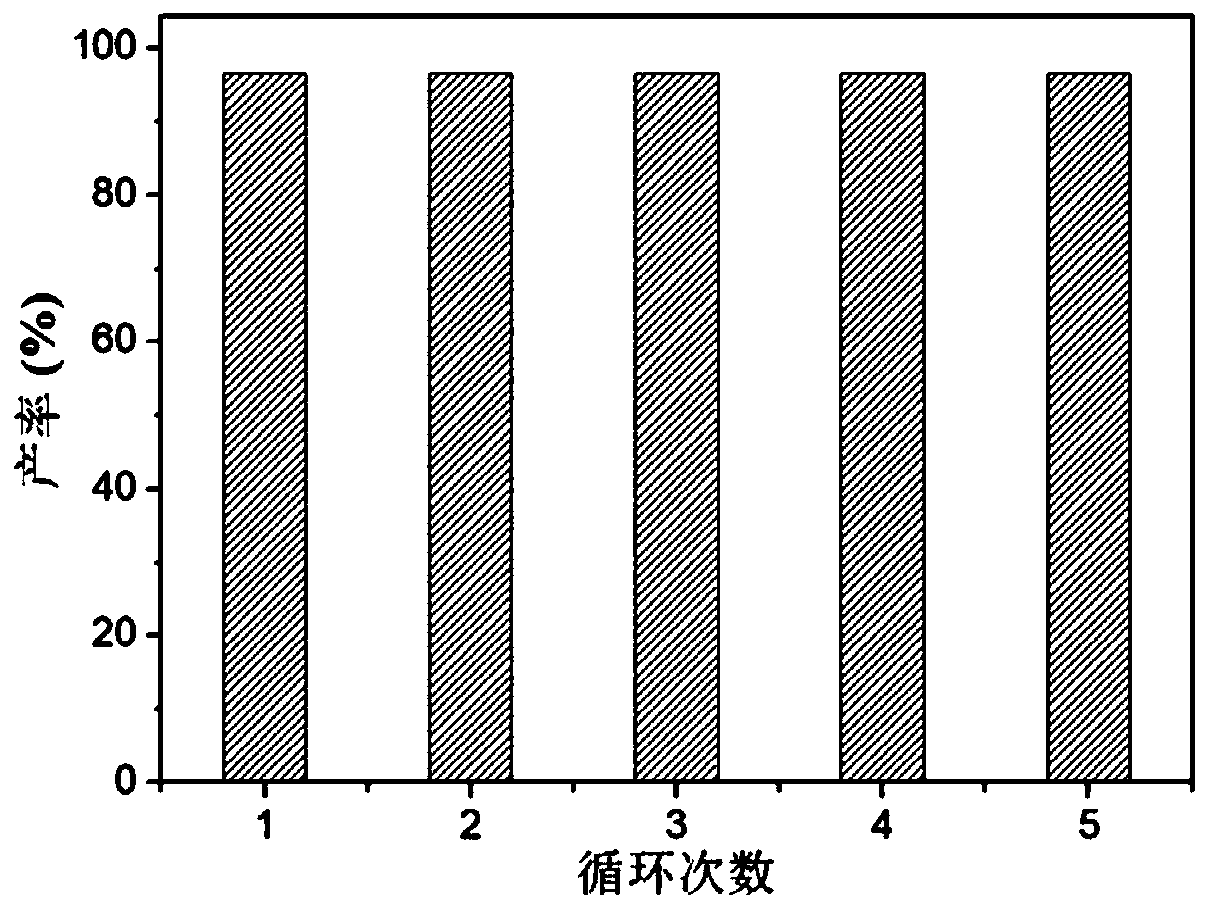
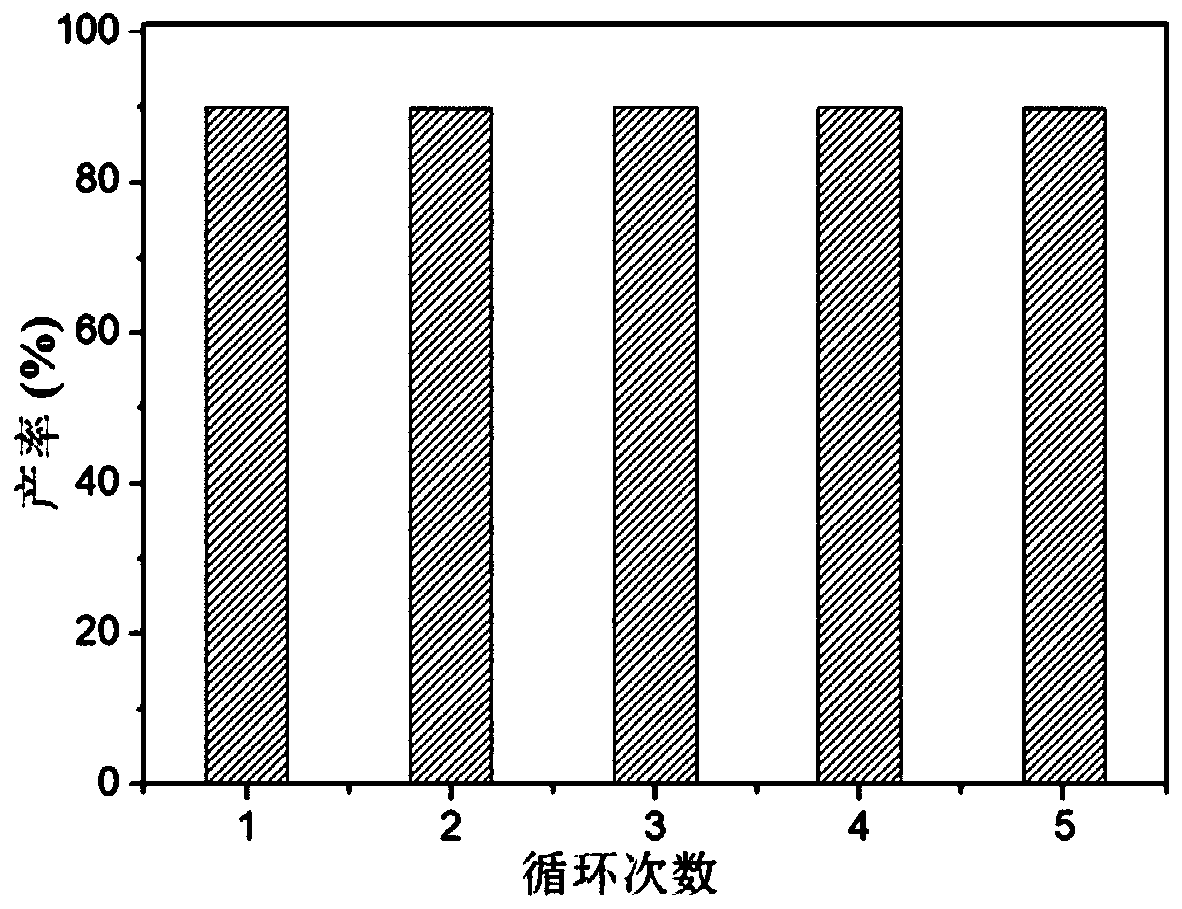
Supported cluster catalyst and preparation and application thereof
ActiveCN110404587AThe synthesis method is simpleIncrease profitOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsOrganic chemistryTrifluoromethyl ketone
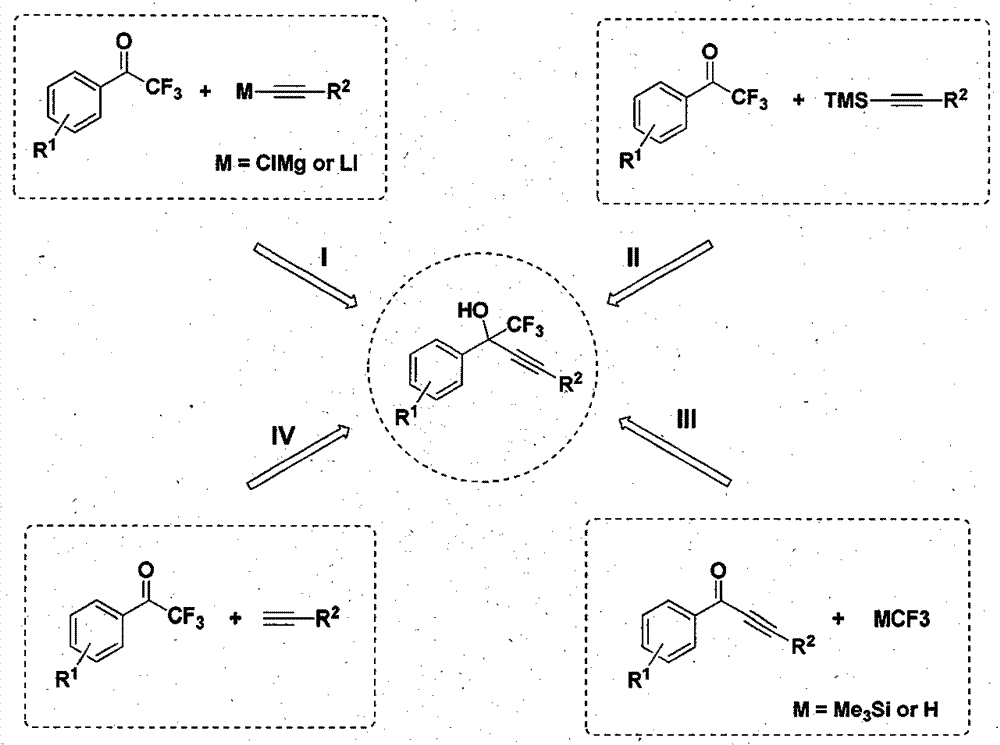
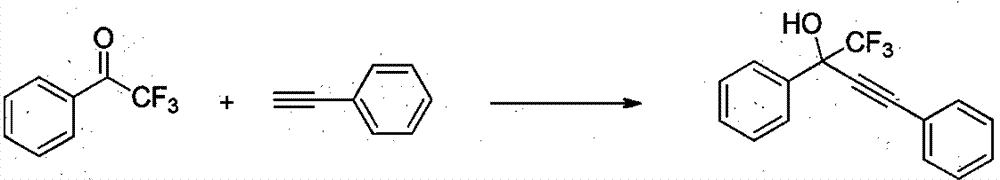
The invention discloses a supported cluster catalyst and preparation and application thereof. The molecular formula of the supported cluster catalyst is Au1Ag24 / ZnO or Au12Ag32 / ZnO, wherein the molecular formula of a Au1Ag24 cluster is Au1Ag24(SPhMe2)18(PPh4) which is called Au1Ag24 for short; the molecular formula of a Au12Ag32 cluster is Au12Ag32(SPhF2)30(PPh4)4 which is called Au12Ag32 for short. The Au1Ag24 / ZnO catalyst and the Au12Ag32 / ZnO catalyst can catalyze an alkynylation reaction of trifluoromethyl ketone at high yield, reaction conditions are mild, the catalyst can be recycled at least five times, and the universality of a substrate is high.
Owner:ANHUI UNIVERSITY +1
Trifluoromethyl substituted continuous quaternary carbon center cyclopropane derivative and synthetic method
ActiveCN105037298AImprove adaptabilityWide adaptabilityOrganic chemistryChemical industryVacuum evaporation
The invention discloses a trifluoromethyl substituted continuous quaternary carbon center cyclopropane derivative and a synthetic method, belonging to the technical field of synthesis of medicine chemical industry. The synthetic method comprises the following steps: adding N-tosyl hydrazone, trifluoromethyl ketone, alkali, a phase transfer catalyst and a solvent into a reactor, carrying out reaction under stirring at 70 to 90 DEG C for 12 to 24 hours, after the reaction is completed, carrying out cooling to room temperature, filtering obtained reaction liquid, carrying out vacuum evaporation and removing the solvent so as to obtain a crude trifluoromethyl substituted continuous quaternary carbon center cyclopropane derivative, and carrying out purifying through column chromatography so as to obtain the trifluoromethyl substituted continuous quaternary carbon center cyclopropane derivative. The method provided by the invention avoids using a transition metal catalyst and uses non-toxic, cheap and easily-available raw materials; the reaction has good adaptability to functional groups and wide adaptability to a substrate, has high product yield and good non-reflect selectivity, can be enlarged to gram-grade scale production and synthesis, and is favorable for industrial production; meanwhile, the obtained trifluoromethyl substituted continuous quaternary carbon center cyclopropane derivative has extensive application in the fields of pesticides, medicines and materials.
Owner:SOUTH CHINA UNIV OF TECH
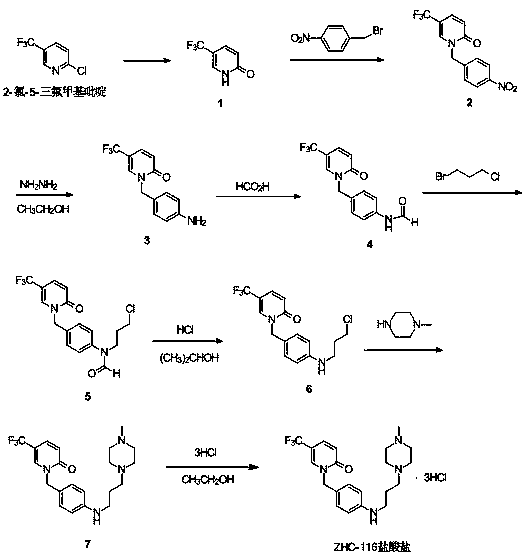
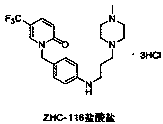
Synthetic method of renal fibrosis resistant medicine 1-(substituted benzyl)-5- trifluoromethyl-2(1H)-pyridone hydrochloride
ActiveCN107652228AReact SafeProcess environmental protectionOrganic chemistryUrinary disorderBenzyl groupPiperazine
The invention relates to a synthetic method of a renal fibrosis resistant medicine 1-((4-(4-methyl piperazine-1-yl)amino)benzyl-5-trifluoromethyl-2(1H)-ketone hydrochloride(ZHC-116 hydrochloride). According to the invention, 2-chloro-5-trifluoromethylpyridine(commercially available) which is used as a raw material undergoes nine synthesis steps to prepare 1-((4-(4-methyl piperazine-1-yl)amino)benzyl-5-trifluoromethyl-2(1H)-ketone. In comparison with existing methods reported in literature, the method of the invention is effective and practical, is adopted to raise total synthetic yield and issuitable for large-scale industrial production.
Owner:GUANGZHOU NANXIN PHARMA +1
Method for preparing chiral beta-trifluoromethyl-beta-hydroxy-alpha-amino acid and derivatives thereof
ActiveCN111269132AImprove biological activitySynthesis shortcutGroup 4/14 element organic compoundsOrganic compound preparationPtru catalystMethyl palmoxirate
The invention relates to a method for preparing chiral beta-trifluoromethyl-beta-hydroxy-alpha-amino acid and derivatives thereof, which specifically comprises the following steps: weighing tert-butylglycinate and derivatives thereof, trifluoromethyl ketone, acid and chiral N-methyl pyridoxal catalyst, adding a solvent, and carrying out a reaction to generate the target product chiral beta-trifluoromethyl-beta-hydroxy-alpha-amino acid and derivatives thereof. Compared with the prior art, the pyridoxal catalyst does not need to protect and deprotect amino, and simple, so that convenient and efficient synthesis of the chiral beta-trifluoromethyl-beta-hydroxyl-alpha-amino acid derivative is achieved.
Owner:SHANGHAI NORMAL UNIVERSITY
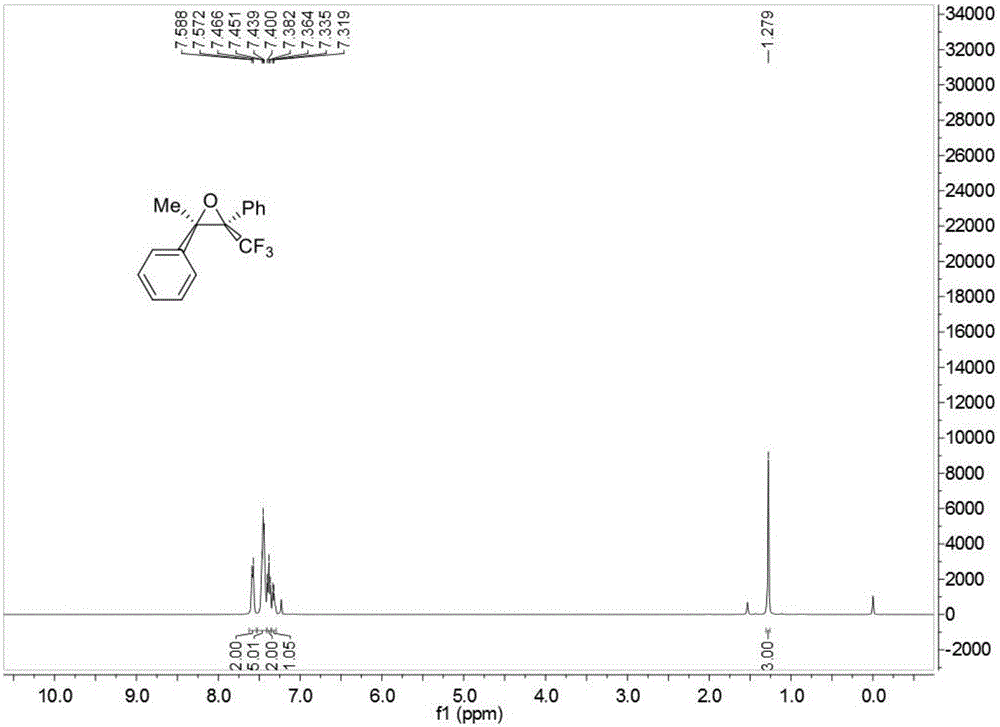
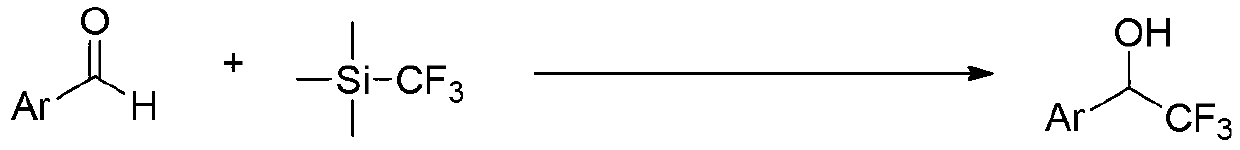
Aromatic ring or heteroaromatic trifluoromethyl ketone compound and preparation method thereof
InactiveCN103214327ASmall equivalentWide range of usesCarboxylic acid nitrile preparationOrganic compound preparationAlcoholOrganic synthesis
The present invention discloses an aromatic ring or a heteroaromatic trifluoromethyl ketone compound and a preparation method thereof, and belongs to the technical field of organic synthesis. In the present invention, aromatic ring or heteroaromatic formaldehyde and trifluoromethyl trimethylsilane as raw materials to synthesize the corresponding a substituted trifluoromethyl alcohol compound, then the trifluoromethyl alcohol compound is oxidized to obtain an aromatic ring or a heteroaromatic trifluoromethyl ketone compound. The method is simple to operate, easy in purification, high in yield, and small in needed oxidant equivalent, and has a wide promotional value. The trifluoromethyl ketone compound prepared by the invention can used as an inhibitor, also is an important intermediate for synthesizing trifluoromethyl-substituted heterocyclic compounds and other compounds, and is a monomer for novel polymer materials. The trifluoromethyl ketone compound prepared by the present invention therefore has wide application range.
Owner:TETRANOV PHARMA CO LTD
Fluorine-containing alpha, beta-unsaturated ketone and synthetic method thereof
InactiveCN101104581AImprove responseReduce pollutionOrganic compound preparationCarbonyl compound preparationFuranSynthesis methods
The invention relates to alpha, beta-unsaturated trifluoromethyl ketones and synthesis method thereof. The structure of the compound is showed in the right. Substituents Ar are 4- methyl-phenyl, 4-methoxyphenyl and 2-furan group. The steps of the method are that: dissolve beta, beta-erythrene (trifluoroacetyl group) derivatives and alkali K3PO4-3H2O in tetrahydrofuran according to a mol ratio of 1:1.5-3; stir the mixed solution under room temperature until the beta, beta-erythrene( trifluoroacetyl group) derivatives completely dissolve and then column-chromatograph the solution, in this way, pure alpha and beta-unsaturated trifluoromethyl ketones can be obtained. The invention takes simple alkali and beta, beta-erythrene (trifluoroacetyl group) derivatives which are easy to get as raw materials, reaction effect is good and side product is little with little pollution and high selectivity, thereby the method is an important way to synthesize alpha, beta-unsaturated trifluoromethyl ketones.
Owner:SHANGHAI UNIV
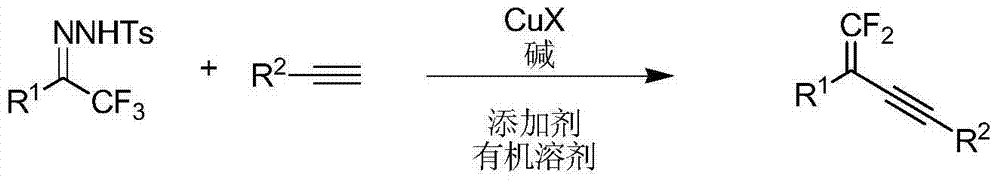
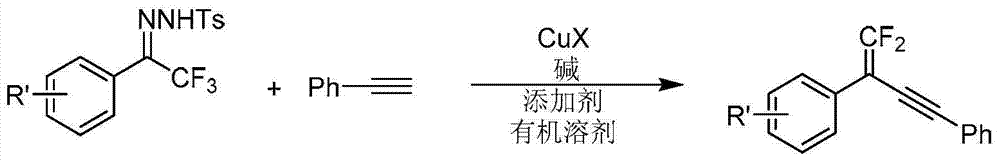
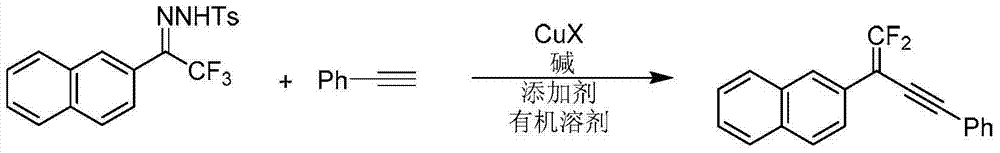
Synthetic method of 1, 1-2 fluorine - 1, 3 - acetylene compounds
ActiveCN104844410AStable and cheap to useImprove universalityGroup 4/14 element organic compoundsAmino preparation from aminesOrganic solventCopper iodide
The invention discloses a synthetic method of 1, 1-2 fluorine - 1, 3 - acetylene compounds. The synthetic method enable tosylhydrazones and acetylene compounds of Alpha, alpha, alpha, 3 methyl ketone to react in organic solvent. 1, 1-2 fluorine - 1, 3 - acetylene compounds is obtained through the catalysis of cuprous CuX and the existing of soda and annexing agent. The synthetic method of 1, 1-2 fluorine - 1, 3 - acetylene compounds preferentially uses copper iodide which is cheap and stable as catalyst, and the operation is relatively convenient and easy. Also the copper iodide has good tolerance and universality to functional groups. The invention has the advantages that the raw materials are easy to obtain, the reaction cost is relative low, and the reaction time is relative short. The invention is suitable for the preparation of 1, 1-2 fluorine - 1, 3 - acetylene compounds.
Owner:PEKING UNIV
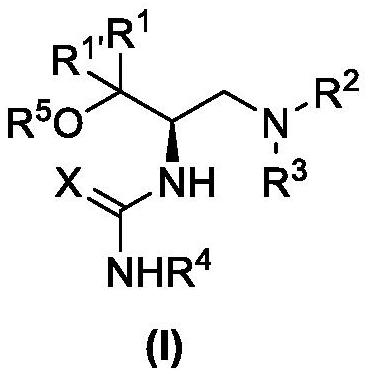
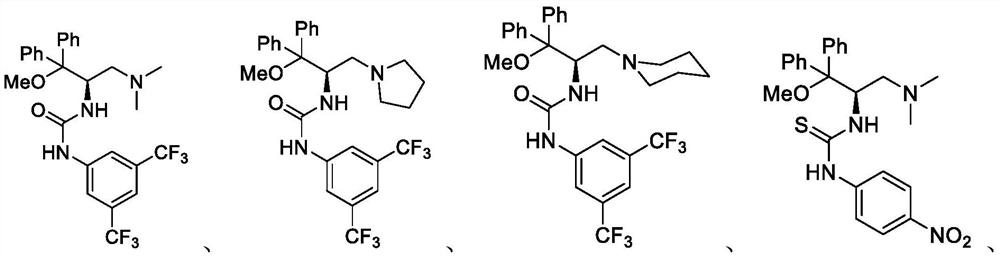
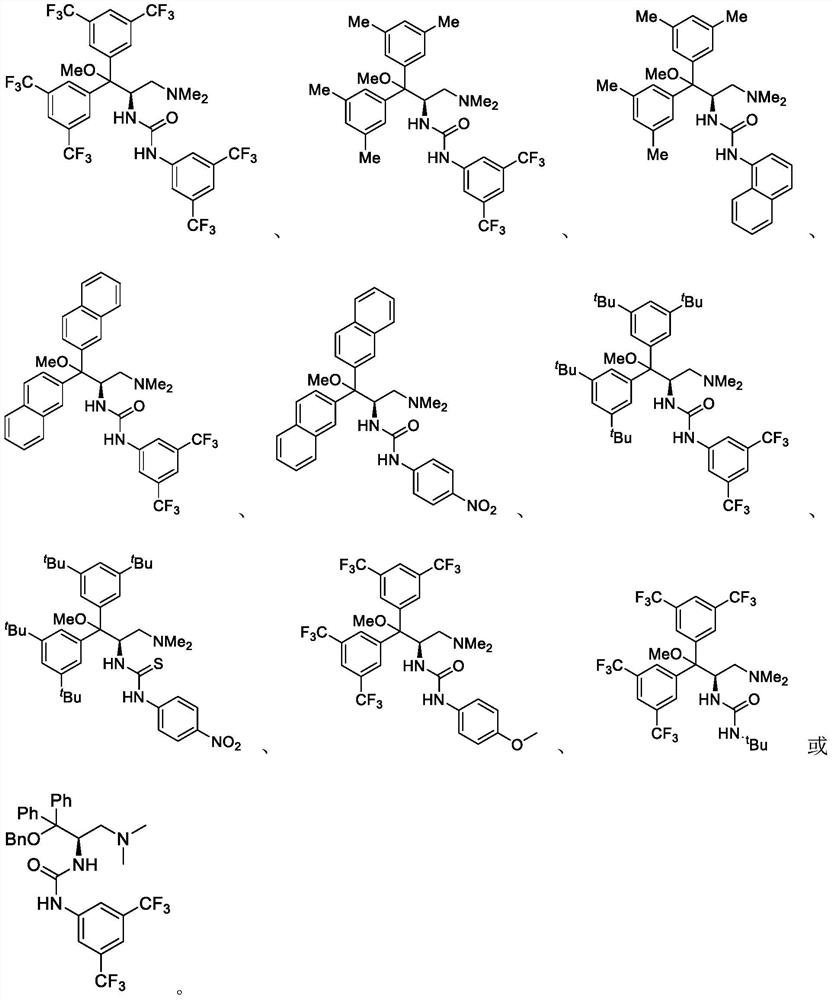
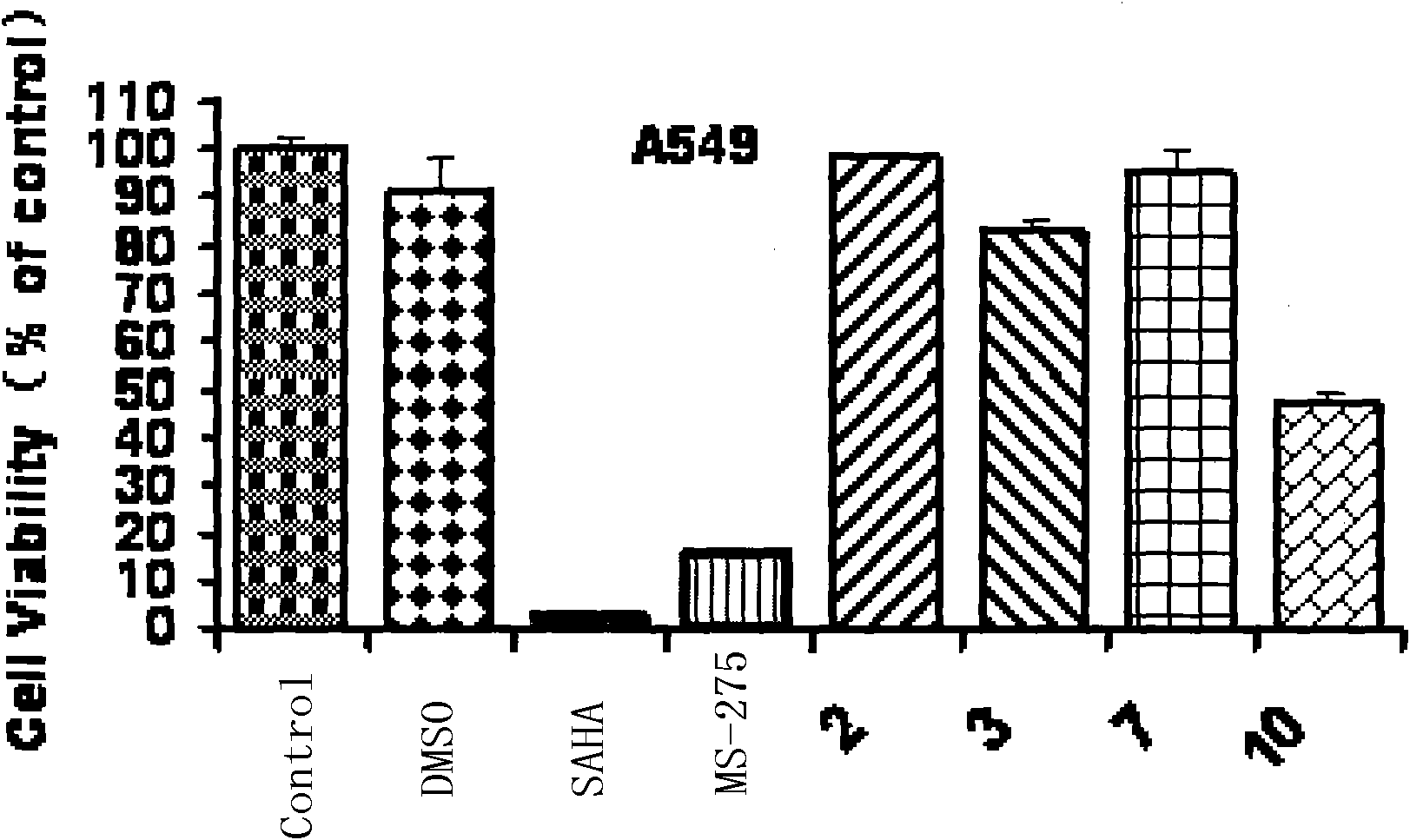
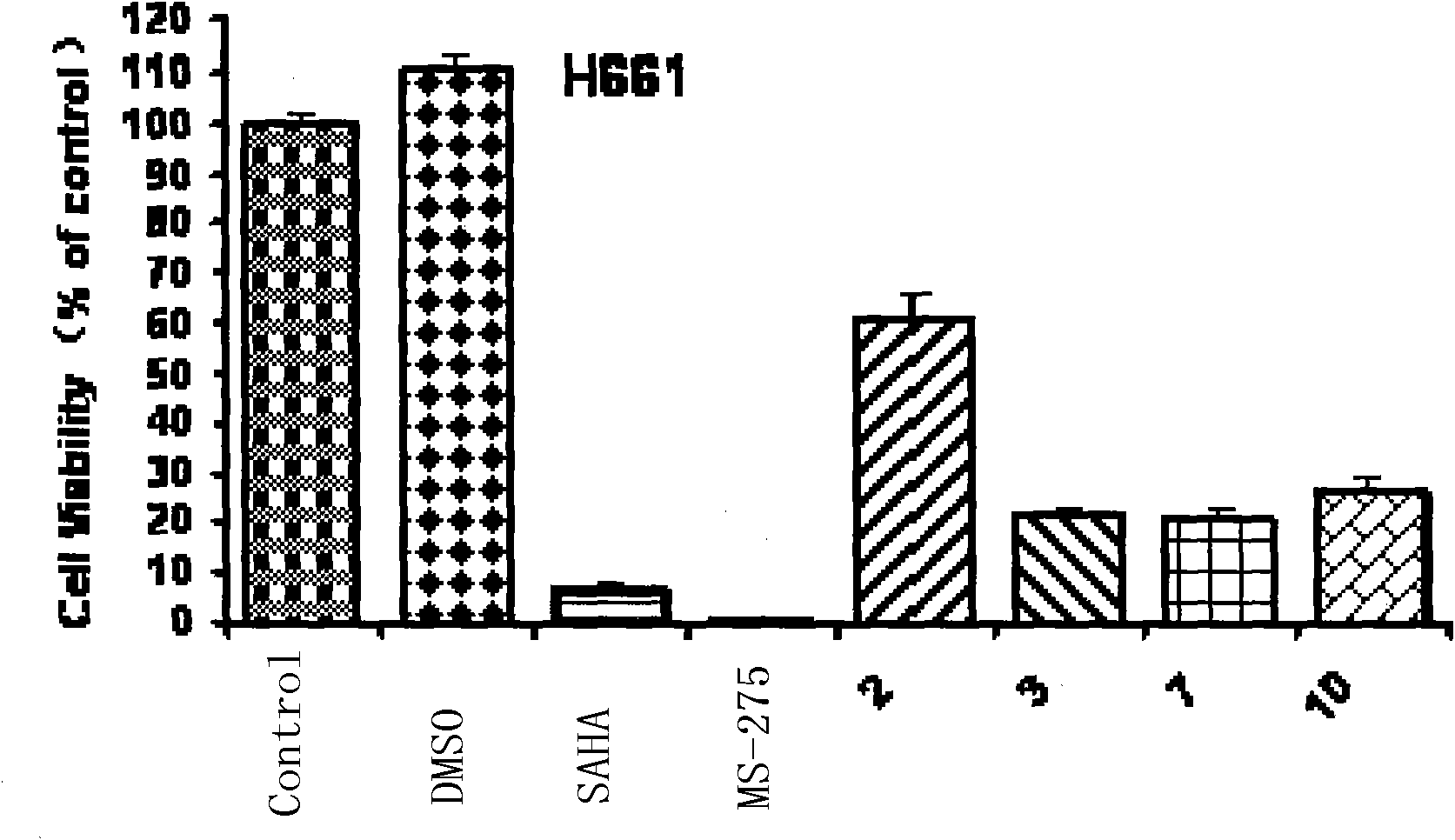
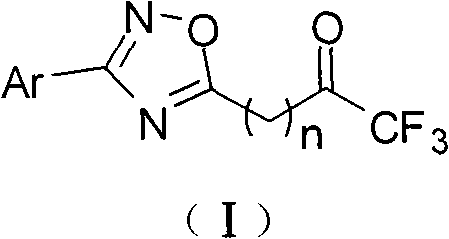
Trifluoromethyl ketone compound used as histone deacetylase inhibitor and application thereof
The invention discloses a compound in the formula (I) or pharmaceutically acceptable salt thereof. Ar is aryl or heterocyclic group and is substituted by optional one or more of the following groups:C1-8 alkyl, C1-8 alkoxy, halogen, nitro , C1-8 aminoalkyl, C1-8 alkyl amino group, C1-8 thio-alkyl, C1-8 halogenated alkyl, C1-8 halogenated alkoxy, C1-8 ester group, phenyl or heterocyclic group. n is an integer from 0 to 8. The drugs prepared by the compound can be used for treating solid tumors or leukemia correlating with cell differentiation or proliferation.
Owner:苏州东南药业股份有限公司
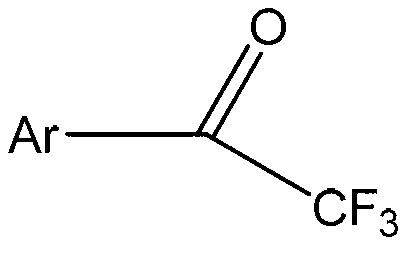
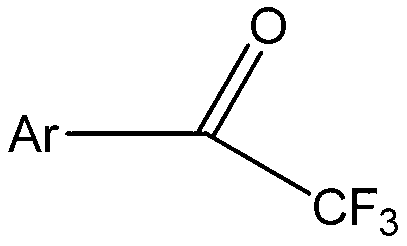
Method for expedient synthesis of [18F]-labeled alpha-trifluoromethyl ketones
InactiveUS6872855B1Satisfies needIn-vivo radioactive preparationsOrganic compound preparationPresent methodImaging agent
The present invention is directed to a convenient method of synthesizing radiolabeled α-trifluoromethyl ketones by a fluorination reaction. The present invention also relates to imaging agents and markers for identifying cell proliferation, or viral infection. The markers and imaging agents including the radiolabeled α-trifluoromethyl ketones that are prepared by the present method.
Owner:UNIV OF SOUTHERN CALIFORNIA
Heterocycle-containing trifluoromethyl ketone compound and preparation method thereof
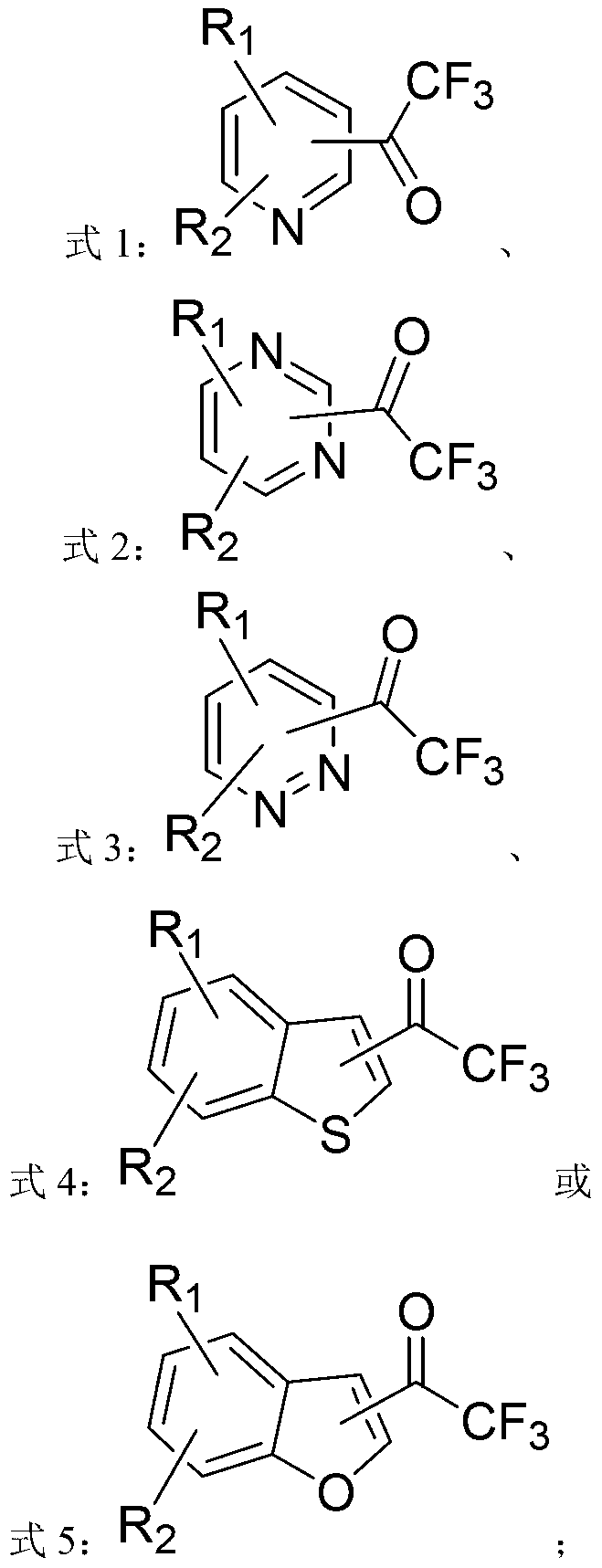
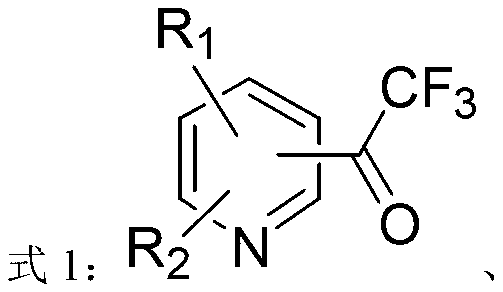
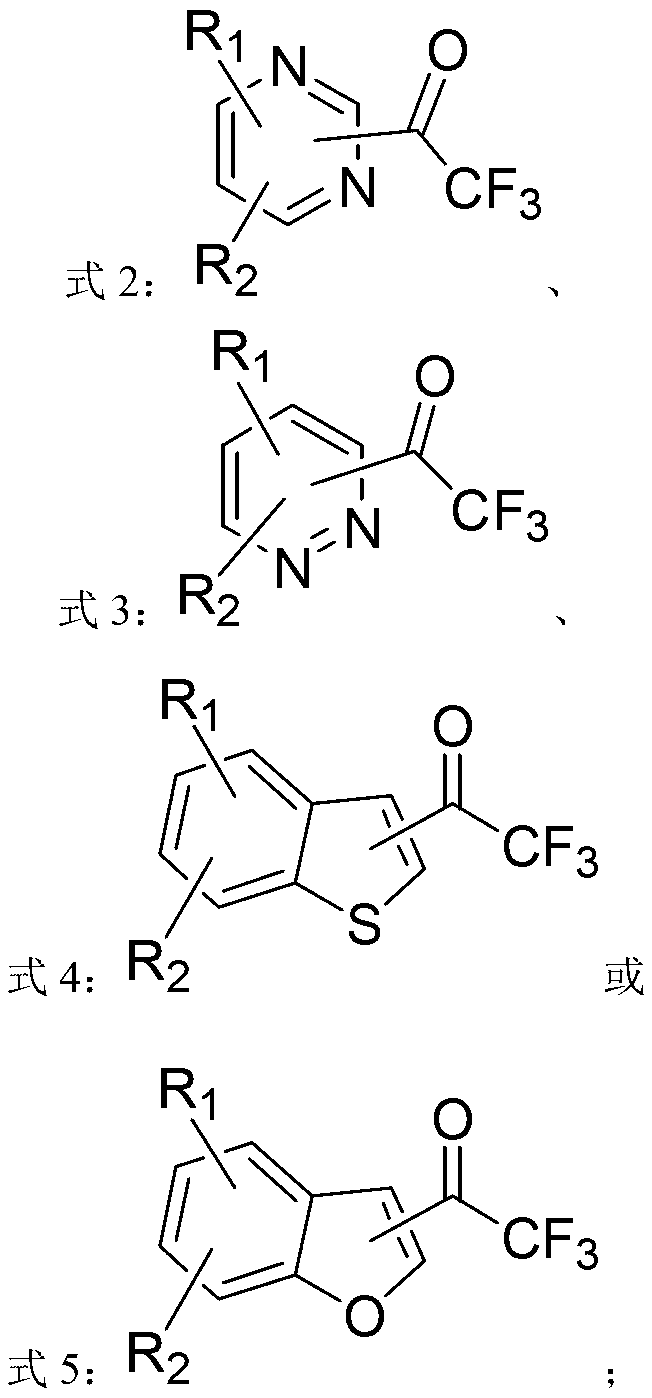
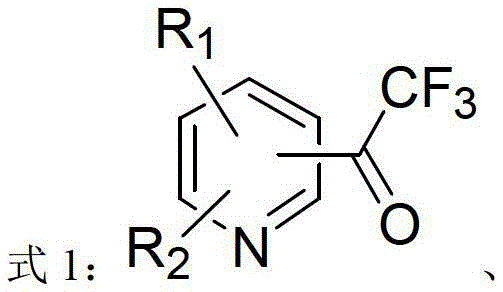
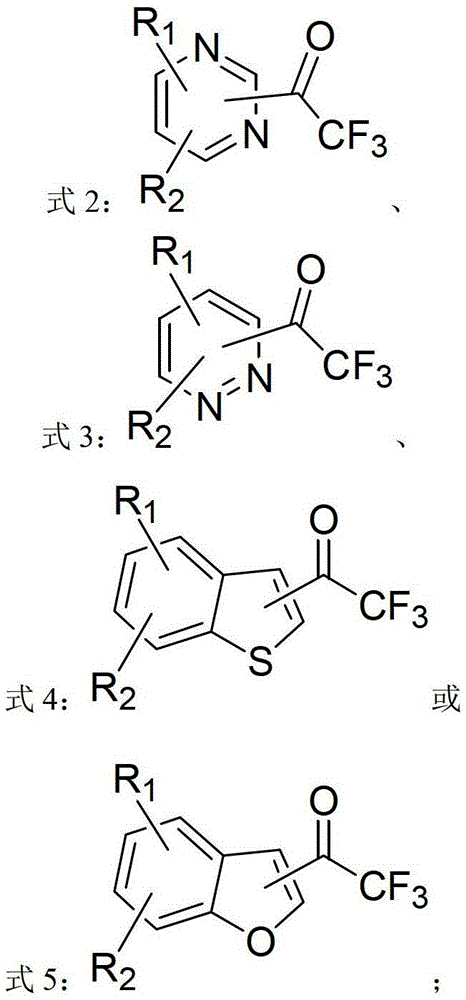
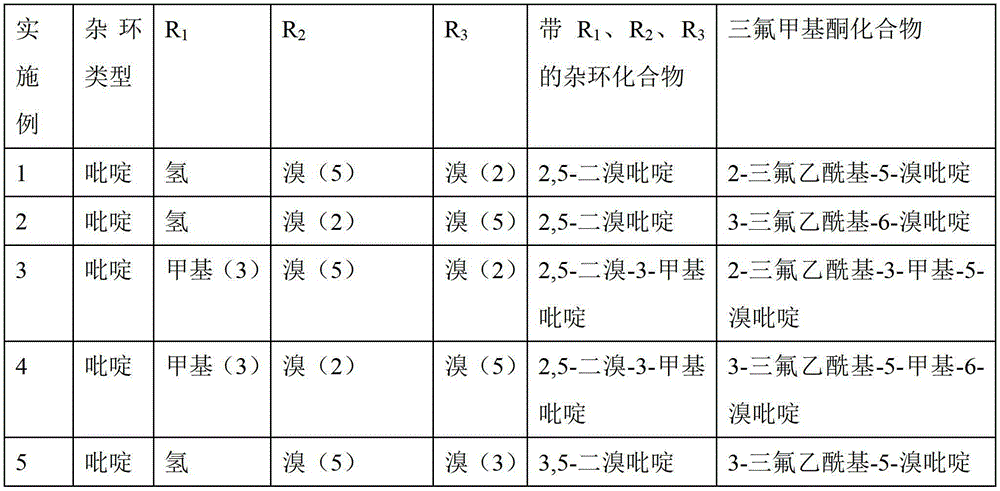
ActiveCN103214413AWide range of usesEasy to operateOrganic chemistryOrganolithium compoundsPyridazine
The invention discloses a heterocycle-containing trifluoromethyl ketone compound and a preparation method thereof. The trifluoromethyl ketone compound is a heterocyclic compound having R1, R2 and trifluoroacetyl. The heterocycle of the heterocyclic compound is of pyridine, pyrimidine, pyridazine, benzothiophene or benzofuran. The preparation method includes: 1) dissolving the heterocyclic compound containing R1, R2 and R3 in a solvent, adding an organolithium reagent in the presence of an inert gas, and reacting at -78 to -10 DEG C to get an organolithium intermediate; and 2) adding N-trifluoroacetylmorpholine to the organolithium intermediate in the step 1), reacting at -78-0 DEG C for a period, quenching the reaction by a quencher, and separating the reaction product. The heterocycle-containing trifluoromethyl ketone compound can be used as an enzyme inhibitor. The preparation method of the present invention can synthesize the heterocycle-containing trifluoromethyl ketone compound in one step, is simple to operate and high in yield, and has a wide application range.
Owner:浙江药领医药科技有限公司
Beta-trifluoromethyl-beta-hydroxy substituted cyclohexanone derivative and synthesis method thereof
ActiveCN109608323AImprove adaptabilityWide adaptabilityOrganic compound preparationOrganic chemistry methodsCyclohexanoneOrganic synthesis
The invention belongs to the technical field of organic synthesis and discloses a beta-trifluoromethyl-beta-hydroxy substituted cyclohexanone derivative and a synthesis method thereof. The beta-trifluoromethyl-beta-hydroxy substituted cyclohexanone derivative has a structural formula shown in a first formula. The synthesis method comprises the following steps of: under the conditions of a catalyst, alkali and organic solvent, mixing alpha, beta-unsaturated oxime ester category compounds with trifluoromethyl ketone category compounds, and post-treating reaction product to obtain the beta-trifluoromethyl-beta-hydroxy substituted cyclohexanone derivative. According to the synthesis method, cheap metal copper is used as a catalyst, no ligand is needed, and the used raw materials are cheap andeasy to obtain; the reaction has good adaptability to functional groups, has wide adaptability to substrates, has high product yield, and can be amplified to gram scale production, the operation is simple and safe, the reaction conditions are mild, the method has good industrial application prospect, the obtained product has wide application in the fields of pesticides, medicines and materials.
Owner:SOUTH CHINA UNIV OF TECH
Method for simply and efficiently preparing efavirenz intermediates
InactiveCN104193567AOrganic compound preparationCarboxylic acid esters preparationSimple Organic CompoundsPtru catalyst
The invention relates to a method for simply and efficiently preparing efavirenz intermediates, which belongs to the technical field of organic compound catalytic chemistry. The method is used for the high-yield preparation of a series of efavirenz intermediates by taking copper as a catalyst and taking trifluoromethyl ketone and terminal alkyne as raw materials. The method is simple to operate and does not need any inert gas shielding; and the method is low in cost, and no extra ligand is added. Therefore, the method has a broad application prospect on the aspects of medicine, pesticides, synthesis of organic functional materials, and the like.
Owner:SHIHEZI UNIVERSITY
Alpha-quaternary carbon trifluoromethyl ketone compound and preparation method thereof
InactiveCN110172015ASynthetic economyEfficient synthesisOrganic compound preparationOrganic chemistry methodsAlkyl transferOrganic chemistry
The invention discloses a method for preparing an alpha-quaternary carbon trifluoromethyl ketone compound and the alpha-quaternary carbon trifluoromethyl ketone compound. A trifluoromethyl ketone compound is subjected to alkylation under the action of a catalyst to rapidly and efficiently synthesize the alpha-quaternary carbon trifluoromethyl ketone compound shown in a formula (I). The method forpreparing the alpha-quaternary carbon trifluoromethyl ketone compound has the advantages that the raw materials are cheap and easy to obtain, the reaction condition is mild, the compatibility of substrate functional groups is high, and the structure of the product can be rapidly expanded. The invention also provides a method for preparing a polyfunctional-group trifluoromethyl synthon.
Owner:SOUTHWEST UNIVERSITY
A method of catalyzing the asymmetric henry reaction of trifluoromethyl ketone
ActiveCN111777530BImprove universalityMild conditionsUrea derivatives preparationOrganic compound preparationPtru catalystCombinatorial chemistry
The invention provides a method for catalyzing the asymmetric Henry reaction of trifluoromethyl ketone. The method of the present invention comprises a brand-new catalyst such as the compound represented by formula I, and has simple operation, high substrate universality, high reaction yield and high enantioselectivity.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Method for preparing trifluoromethyl functionalized 1,3-dioxolane
The invention discloses a method for preparing trifluoromethyl functionalized 1,3-dioxolane. The method comprises the following procedures: subjecting trifluoromethyl ketone and a terminal alkyne compound, which are in the mole ratio of 2: 1, to a stirring reaction for 24 to 36 hours under the conditions of a silver catalyst, an alkaline environment and an organic solvent; and adding saturated common salt water to quench the reaction, carrying out extraction on the reacted mixture for 2 to 3 times by using dichloromethane, mixing organic phases, carrying out rotary evaporation to remove the organic solvent, and carrying out column chromatography separation, thereby obtaining the trifluoromethyl functionalized 1,3-dioxolane. The method disclosed by the invention for simply and efficiently preparing the trifluoromethyl functionalized 1,3-dioxolane is developed for the first time. The method has an extensive application prospect in the aspects of synthesis and the like of medicines, pesticides and organic functional materials.
Owner:SHIHEZI UNIVERSITY
A simple and efficient method for preparing efavirenz intermediates
InactiveCN104193567BOrganic compound preparationCarboxylic acid esters preparationMaterial synthesisAlkyne
The invention relates to a simple and efficient method for preparing an efavirenz intermediate, which belongs to the technical field of organic compound catalytic chemistry. The method uses copper as a catalyst and trifluoromethyl ketone and terminal alkyne as raw materials to prepare a series of efavirenz intermediates in high yield. The method is characterized by simple operation without any inert gas protection; low cost and no need to add any ligands. Therefore, this method will have broad application prospects in the synthesis of medicine, pesticides, and organic functional materials.
Owner:SHIHEZI UNIVERSITY
Method for catalyzing asymmetric Henry reaction of trifluoromethyl ketone
ActiveCN111777530AImprove universalityMild conditionsUrea derivatives preparationOrganic compound preparationPtru catalystCombinatorial chemistry
The invention provides a method for catalyzing asymmetric Henry reaction of trifluoromethyl ketone. The method adopts a brand-new catalyst namely a compound shown as a formula I, and is simple to operate, high in substrate universality, high in reaction yield and high in enantioselectivity.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
A kind of 5-trifluoromethyl-4h-imidazolin-4-one derivative and its synthesis method
ActiveCN108976170BImprove adaptabilityWide adaptabilityOrganic chemistryChemical synthesisPtru catalyst
Owner:SOUTH CHINA UNIV OF TECH
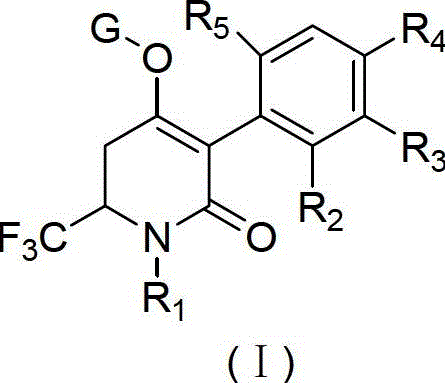
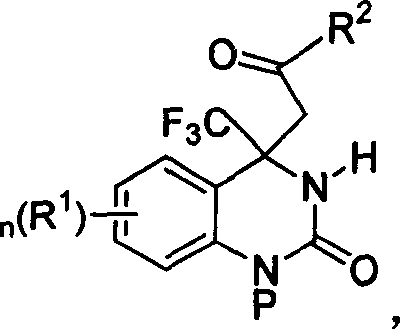
Trifluoromethyl-substituted dihydropyridone derivatives, preparation method and application thereof
The invention discloses a trifluoromethyl substituted dihydropyridine ketone derivative with a structural formula (I) (img file='DDA00002638020300011.TIF' wi = '445' he = '383' / ), and definition of each substituent is shown as in the specifications. The invention also provides a method for preparing the derivative. The derivative provided by the invention can be used for the preparation of agricultural chemical herbicides.
Owner:SINOCHEM LANTIAN +1
Heterocycle-containing trifluoromethyl ketone compound and preparation method thereof
Owner:浙江药领医药科技有限公司
Novel synthesis method of 4, 4, 4-trifluoro-1-butanol and homologue thereof
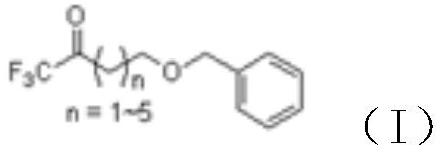
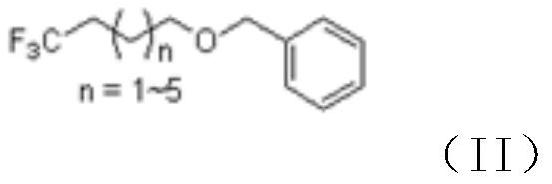
ActiveCN112778089AOrganic compound preparationCarbonyl compound preparationOrganic synthesisGrignard reagent
The invention discloses a novel synthesis method of 4, 4, 4-trifluoro-1-butanol and a homologue thereof, and relates to the technical field of organic synthesis. The method mainly comprises the following steps of: (1) reacting ethyl trifluoroacetate with a Grignard reagent to prepare benzyloxy substituted alkyl-trifluoromethyl ketone; (2) carrying out reduction reaction on the benzyloxy substituted alkyl-trifluoromethyl ketone to prepare 1-benzyloxy-trifluorosubstituted alkyl; and (3) carrying out hydrolysis on the 1-benzyloxy-trifluorosubstituted alkyl, so as to prepare 4, 4, 4-trifluoro-1-butanol or a homologue thereof disclosed by the invention. Compared with the prior art, Freon raw materials which can pollute air are not used; dangerous lithium aluminum hydride is not used; high-temperature reaction is not needed; and the raw material ethyl trifluoroacetate is cheap and easily available, and the other main raw material Grignard reagent can also be synthesized from cheap raw materials.
Owner:万知科技股份有限公司
One-pot method for preparing 3-trifluoromethylisoxazole compound
ActiveCN107963996BEasy to operateMild conditionsGroup 4/14 element organic compoundsAlkyneMethyl group
The invention discloses a novel one-pot method for preparing 3-trifluoromethyl-substituted isoxazole compounds. The method is to prepare fluorodiazomethane from commercially available trifluoroethylamine, and then It is obtained by coupling reaction with alkyne compounds under the catalysis of cheap copper; the method is simple to operate, mild in reaction conditions, low in cost, less by-products, high in yield, high in functional group tolerance, and can scale up the reaction. At the same time, a more in-depth mechanism study was made, and the mechanism of the intermediate of trifluoromethyl ketoxime compound in the reaction was proposed.
Owner:JIANGXI NORMAL UNIV
A kind of visible light catalyzed preparation method of α-aryl-β-trifluoromethyl ketone compound
ActiveCN108774121BImprove toleranceLow priceOrganic compound preparationCarbonyl compound preparationMeth-Ptru catalyst
The invention discloses a method for preparing α-aryl-β-trifluoromethyl ketones by visible light catalysis. Sodium phosphate was used as a raw material, and under the synergistic effect of photocatalyst, visible light, solvent and oxygen donor, α-aryl-β-trifluoromethyl ketones were synthesized. The present invention discloses a new method for preparing β-trifluoromethyl-α-aryl ketone by performing visible-light photocatalytic reaction of a series of α-monoaryl allyl alcohol raw materials and Langlois reagent under mild reaction conditions method of compounds. The catalyst system has good tolerance to various synthetically useful functional groups. The method of the present invention does not need any strong oxidizing agent and transition metal catalyst, and uses easily available and relatively low-cost sodium trifluoromethanesulfinate as a starting material, further increasing the application of the synthesis method in organic synthesis.
Owner:MINNAN NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
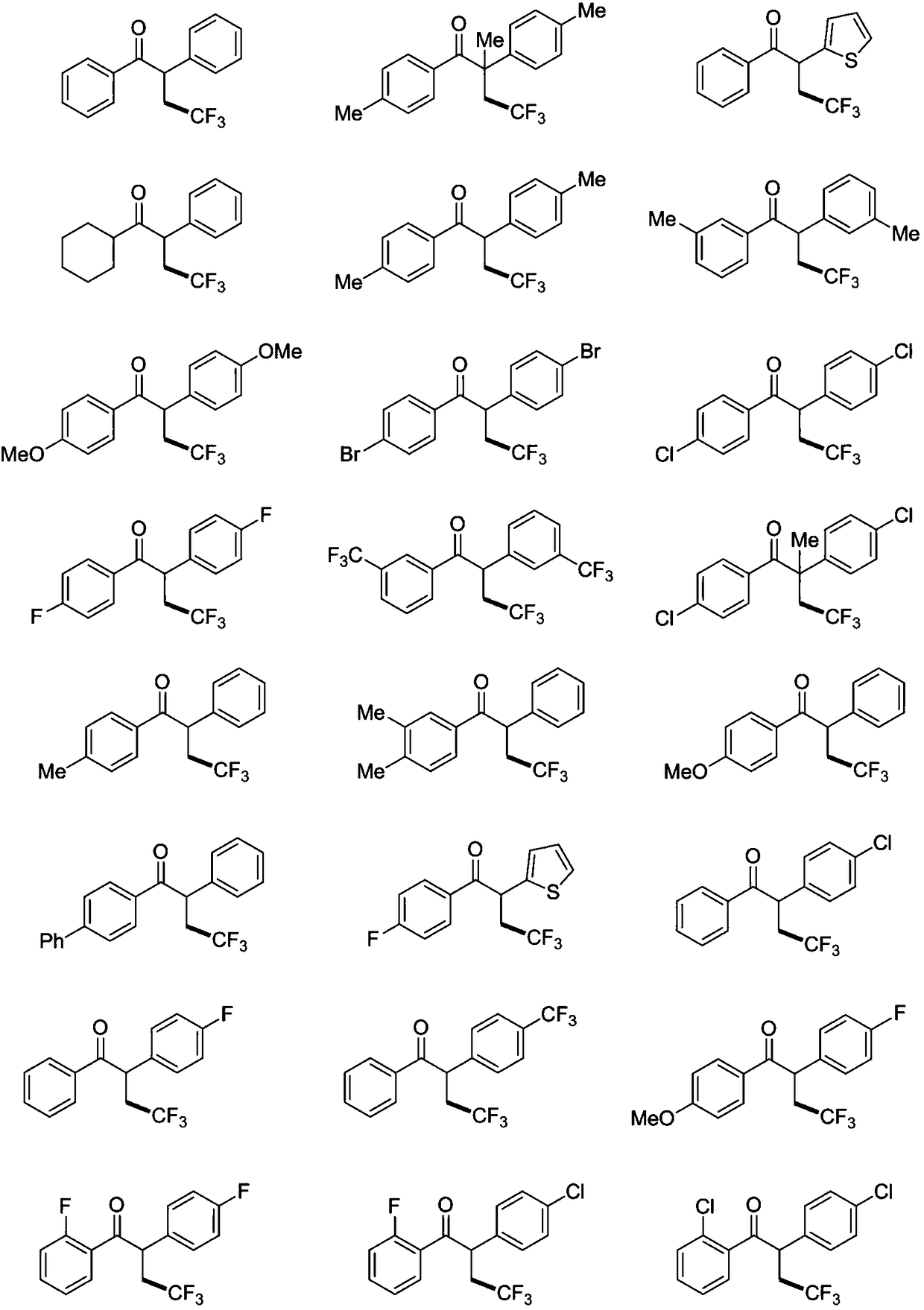
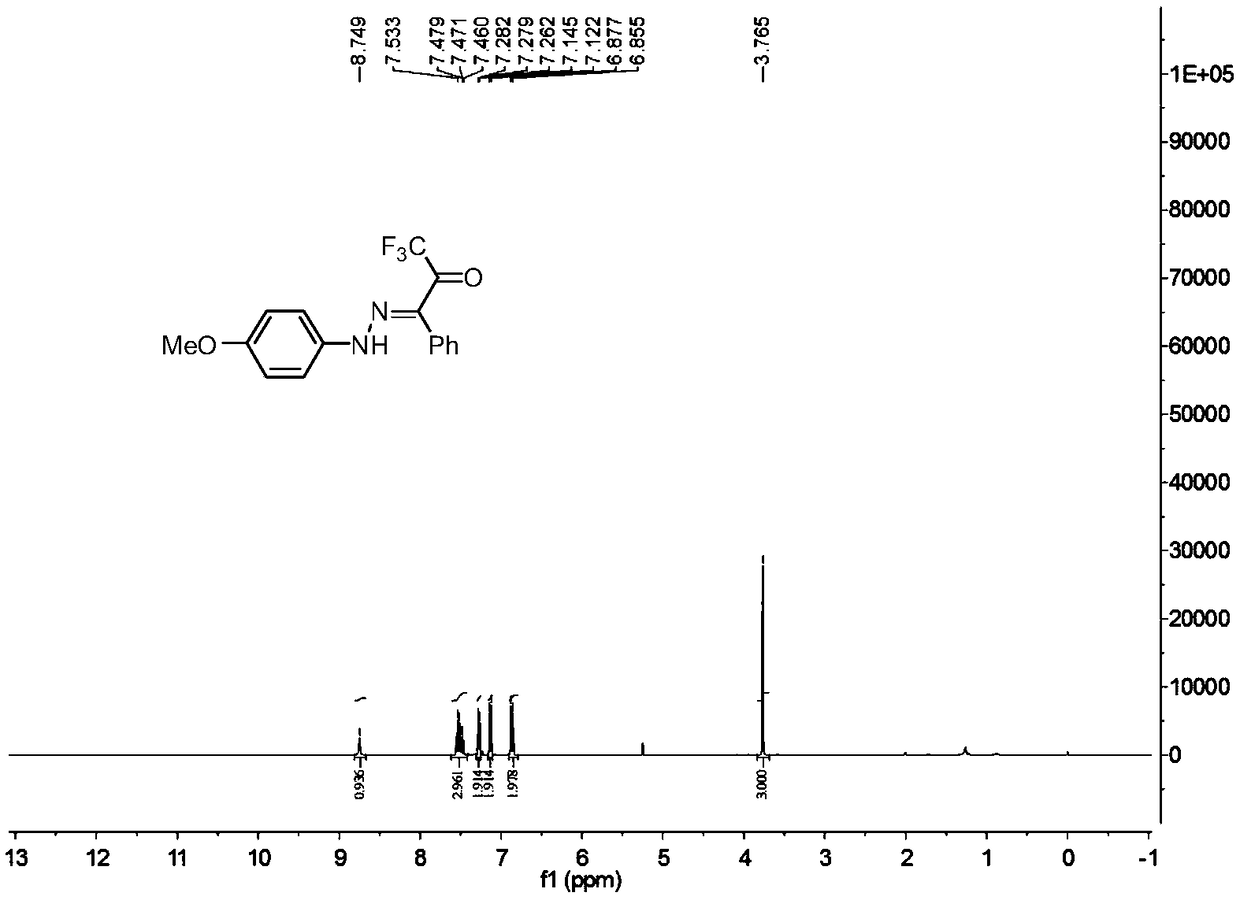
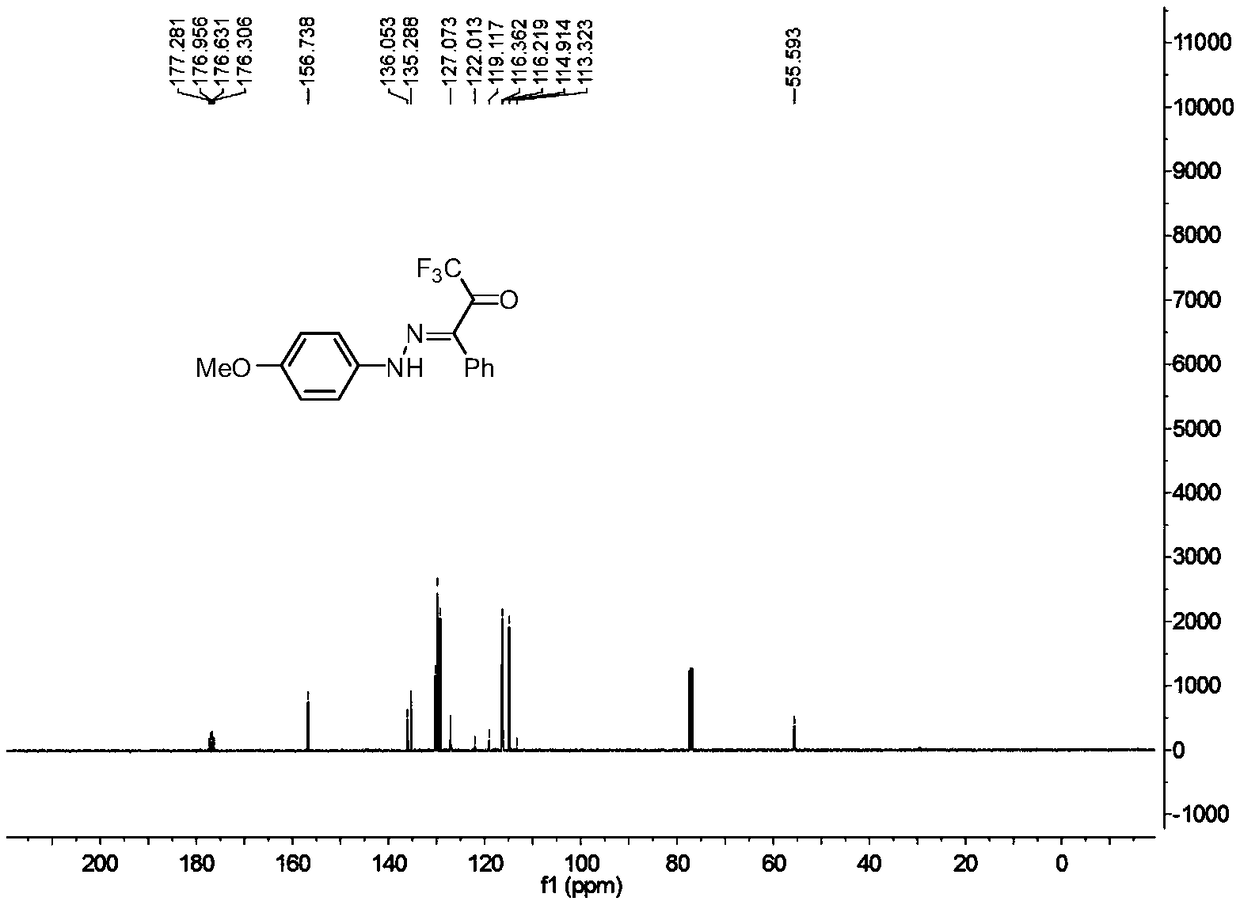
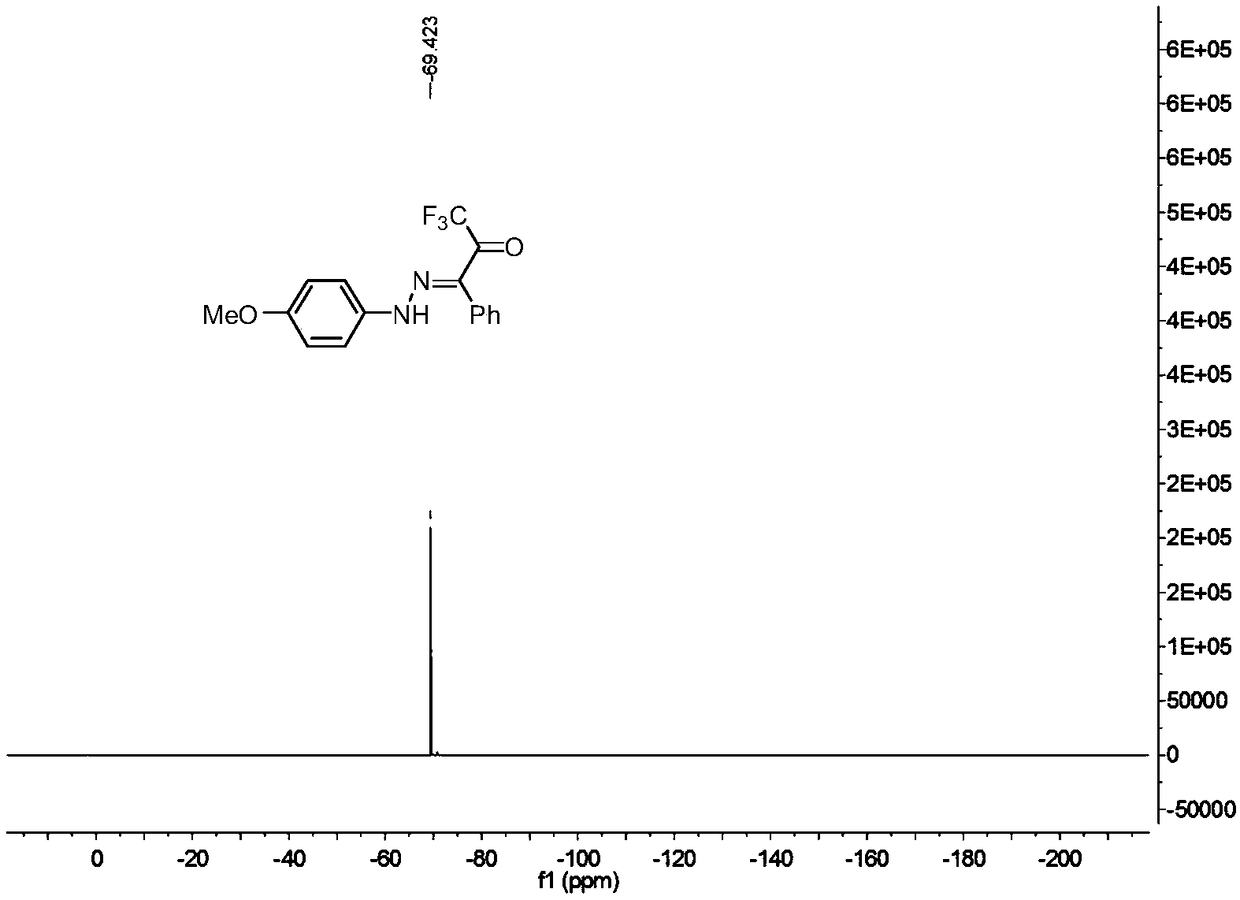
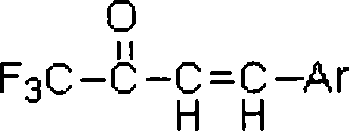
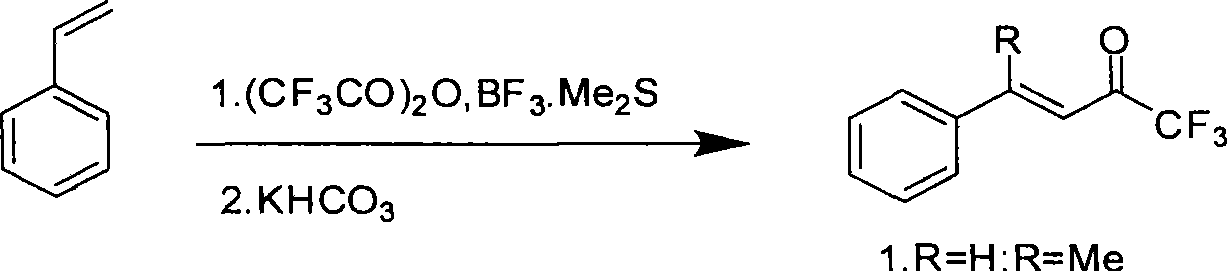
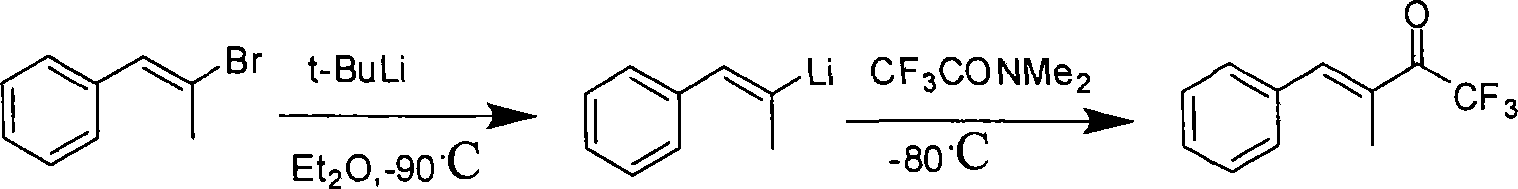
![Method for expedient synthesis of [18F]-labeled alpha-trifluoromethyl ketones Method for expedient synthesis of [18F]-labeled alpha-trifluoromethyl ketones](https://images-eureka.patsnap.com/patent_img/0b48dbb3-142e-46aa-ab5c-f0695d583427/US06872855-20050329-D00001.png)
![Method for expedient synthesis of [18F]-labeled alpha-trifluoromethyl ketones Method for expedient synthesis of [18F]-labeled alpha-trifluoromethyl ketones](https://images-eureka.patsnap.com/patent_img/0b48dbb3-142e-46aa-ab5c-f0695d583427/US06872855-20050329-D00002.png)
![Method for expedient synthesis of [18F]-labeled alpha-trifluoromethyl ketones Method for expedient synthesis of [18F]-labeled alpha-trifluoromethyl ketones](https://images-eureka.patsnap.com/patent_img/0b48dbb3-142e-46aa-ab5c-f0695d583427/US06872855-20050329-D00003.png)