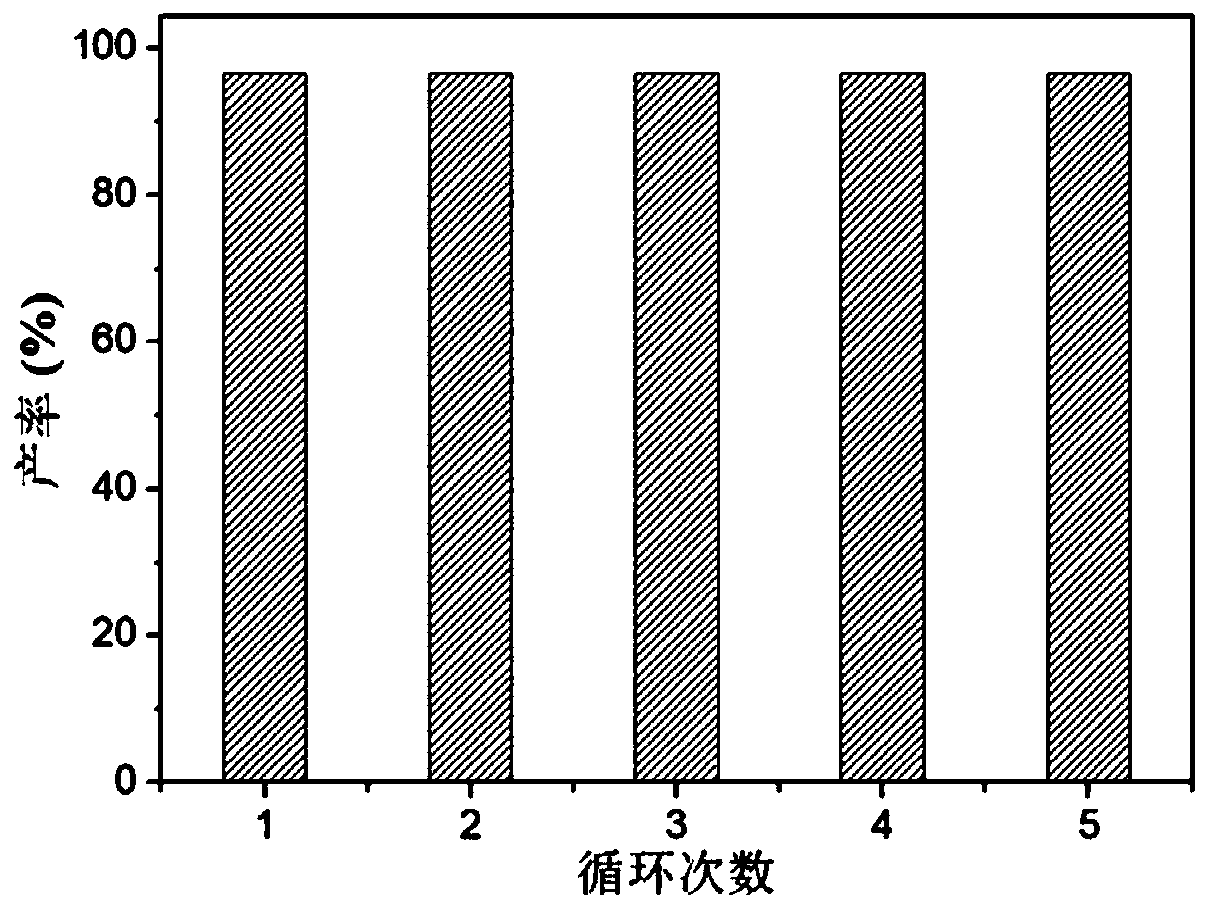
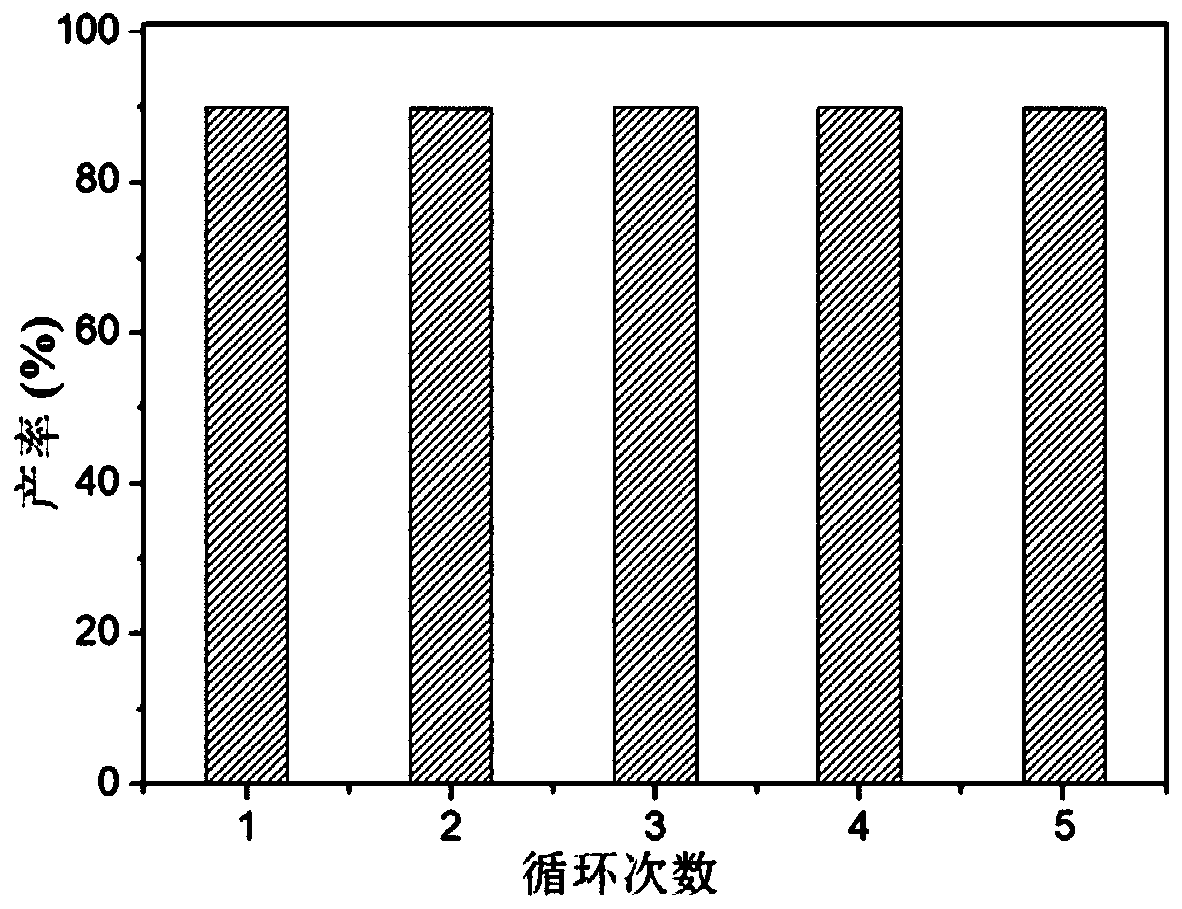
Supported cluster catalyst and preparation and application thereof
A catalyst and supported technology, applied in the preparation of carbon-based compounds, the preparation of organic compounds, physical/chemical process catalysts, etc., can solve the problems of large substrate limitations, large catalyst dosage, long reaction time, etc., and achieve utilization High, simple synthesis method, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Au 12 Ag 32 / ZnO catalyzed the reaction of phenylacetylene with 2,2,2,-trifluoroacetophenone in DMSO solution.
[0030] Au 12 Ag 32 / ZnO catalyst (30mg), phenylacetylene (0.5mmol), 2,2,2,-trifluoroacetophenone (0.75mmol), K 2 CO 3 (0.24mmol) and DMSO (500μL) were added into a dry Schlenk flask, under the protection of argon, the reaction mixture was stirred at 50°C for 12h, and the reaction solution and solid catalyst were separated by centrifugation. Extract the target product in the reaction solution with saturated brine and dichloromethane, collect CH 2 Cl 2 anhydrous Na 2 SO 4 After drying, the crude product was purified by silica gel column chromatography (EtOAc / PE=1:20) to give the main product in 87.7% yield.
[0031] 1 H NMR (400MHz, CDCl 3 )δ7.83~7.81(m,2H),7.53(d,J=8.0Hz,2H),7.45~7.34(m,6H), 3.13(s,1H)ppm; 13 C NMR (101MHz, CDCl 3 )δ134.26, 131.04, 128.53, 127.45, 127.23, 126.17, 123.78, 120.94, 119.92, 87.04, 83.40, 72.32ppm.
Embodiment 2
[0032] Example 2: Au 12 Ag 32 / ZnO catalyzed the reaction of phenylacetylene with 2,2,2,-trifluoroacetophenone in DMF solution.
[0033] Au 12 Ag 32 / ZnO catalyst (30mg), phenylacetylene (0.5mmol), 2,2,2,-trifluoroacetophenone (0.75mmol), K 2 CO 3 (0.24mmol) and DMF (500μL) were added into a dry Schlenk flask, under the protection of argon, the reaction mixture was stirred at 50°C for 12h, and the reaction solution and solid catalyst were separated by centrifugation. Extract the target product in the reaction solution with saturated brine and dichloromethane, collect CH 2 Cl 2 anhydrous Na 2 SO 4 After drying, the crude product was purified by silica gel column chromatography (EtOAc / PE=1:20) to give the main product in 96.7% yield.
Embodiment 3
[0034] Example 3: Au 12 Ag 32 / ZnO catalyzed the reaction of phenylacetylene with 2,2,2,-trifluoroacetophenone in acetonitrile solution.
[0035] Au 12 Ag 32 / ZnO catalyst (30mg), phenylacetylene (0.5mmol), 2,2,2,-trifluoroacetophenone (0.75mmol), K 2 CO 3 (0.24mmol) and acetonitrile (500μL) were added into a dry Schlenk flask, under the protection of argon, the reaction mixture was stirred at 50°C for 12h, and the reaction solution and solid catalyst were separated by centrifugation. The solvent in the reaction solution was removed by a rotary evaporator, and the crude product was purified by silica gel column chromatography (EtOAc / PE=1:20) to obtain the main product with a yield of 19.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com