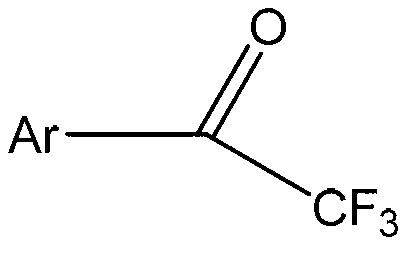
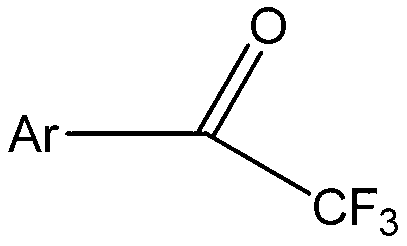
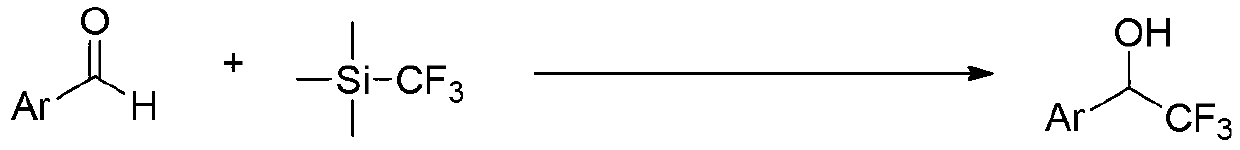
Aromatic ring or heteroaromatic trifluoromethyl ketone compound and preparation method thereof
A technology of trifluoromethyl ketone and trifluoromethyl trimethylsilicon, which is applied in the field of organic synthesis, can solve the problems of complex post-processing, low yield, large oxidant equivalent, etc., and achieves easy purification, high yield, and oxidant. small equivalent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The aromatic ring or aromatic heterocyclic trifluoromethyl ketone compound in this embodiment is trifluoroacetophenone, and its structural formula is:
[0029]
[0030] The preparation method of the trifluoroacetophenone of the present embodiment may further comprise the steps:
[0031] 1) Add benzaldehyde, K 2 CO 3 Dissolve in DMF according to the molar ratio of 20:1, and put it into a three-neck glass bottle together with the magnet, and repeatedly vacuumize and flush it into N 2 After 4 times, place the three-neck glass bottle in an ice bath and stir vigorously; the amount of DMF is to add 3L of DMF per mole of benzaldehyde;
[0032] 2) When the temperature in step 1) drops to 0°C, slowly add trifluoromethyl trimethyl silicon dropwise according to the benzaldehyde:trifluoromethyl trimethyl silicon molar ratio of 1:1; After 30 min of fluoromethyl ketone, the temperature was raised to room temperature for 0.5 h of reaction;
[0033] 3) Slowly add 5 mL of 4M HCl ...
Embodiment 2
[0039] The aromatic ring or aromatic heterocyclic trifluoromethyl ketone compound in this embodiment is p-methyl trifluoroacetophenone, and its structural formula is:
[0040]
[0041] The preparation method of the p-methyltrifluoroacetophenone of the present embodiment comprises the following steps:
[0042] 1) P-tolualdehyde, K 2 CO 3 Dissolve in DMF according to the molar ratio of 20:1, and put it into a three-neck glass bottle together with the magnet, and repeatedly vacuumize and flush it into N 2 After 4 times, the three-neck glass bottle was placed in an ice bath and stirred vigorously; the amount of DMF was 4L of DMF per mole of p-tolualdehyde;
[0043] 2) When the temperature in step 1) drops to -5°C, slowly add trifluoromethyl trimethyl silicon dropwise according to the molar ratio of p-tolualdehyde: trifluoromethyl trimethyl silicon; After dropping the trifluoromethyl ketone for 15 minutes, the temperature was raised to room temperature for 48 hours;
[0044]...
Embodiment 3
[0050] The aromatic ring or aromatic heterocyclic trifluoromethyl ketone compound in this embodiment is p-methoxy trifluoroacetophenone, and its structural formula is:
[0051]
[0052] The preparation method of the p-methoxy trifluoroacetophenone of the present embodiment, comprises the following steps:
[0053] 1) P-methoxybenzaldehyde, Li 2 CO 3 Dissolve in DMF according to the molar ratio of 20:1, and put it into a three-neck glass bottle together with the magnet, and repeatedly vacuumize and flush it into N 2 After 4 times, the three-neck glass bottle was placed in an ice bath and stirred vigorously; the amount of DMF was 5L of DMF per mole of p-methoxybenzaldehyde;
[0054] 2) When the temperature in step 1) drops to -3°C, slowly add trifluoromethyl trimethyl silicon dropwise according to the molar ratio of p-methoxybenzaldehyde: trifluoromethyl trimethyl silicon 1:2.5 ; After dropping the trifluoromethyl ketone for 20 minutes, the temperature was raised to room te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com