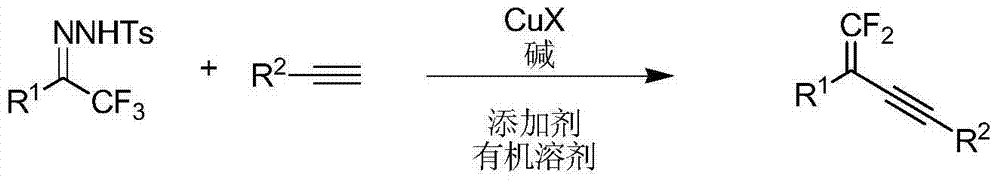
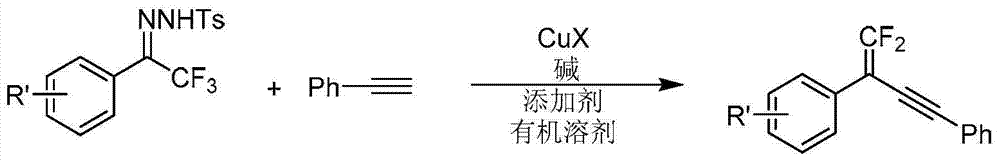
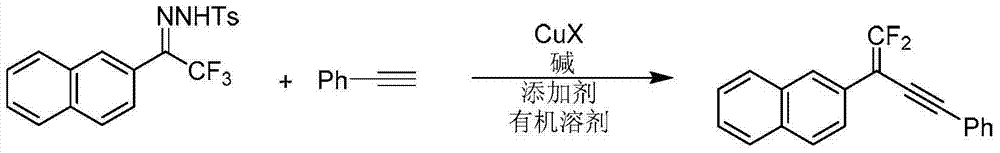
Synthetic method of 1, 1-2 fluorine - 1, 3 - acetylene compounds
A synthesis method and compound technology, which are applied in the field of synthesis of 1,1-difluoro-1,3-enyne compounds, can solve problems such as low practicability, achieve good universality, high efficiency, stable and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 2,4-Diphenyl 1,1-difluoro-1-en-3-yne
[0049]Take a 10mL Schlenk tube, put it into a magnet stopper, put a rubber stopper on it, heat and dry it, and cool it under nitrogen, then add 68mg (0.2mmol ), lithium tert-butoxide 32mg (0.4mmol), cuprous iodide 8mg (0.04mmol), appropriate amount of additives, nitrogen pumping, then add 3mL 1,4-dioxane, add phenylacetylene 20mg (0.2 mmol). React at a certain temperature on an electromagnetic heating stirrer for about 2 hours. After the reaction, use a 10mL sand core column filled with 3cm high silica gel to filter and elute with an appropriate amount of petroleum ether. The resulting filtrate is removed from the reaction solvent by a rotary evaporator and purified by column chromatography to obtain 2,4-diphenyl 1,1-difluoro-1-en-3-yne, its structure is shown in the following formula:
[0050]
[0051] Taking this reaction as an example, under the condition that other conditions remain unchanged, by adjusting the type or rea...
Embodiment 2
[0057] 2-(4’-fluorophenyl)-4-phenyl 1,1-difluoro-1-en-3-yne
[0058] Take a 10mL Schlenk tube, put it into a magnetic sub-plug and put a rubber stopper on it, heat it to dry, cool it under nitrogen, then add 1,1,1-trifluoromethyl-2-(4'-fluorophenyl)ketone and p-toluenesulfonate Acylhydrazone 72mg (0.2mmol), lithium tert-butoxide 32mg (0.4mmol), cuprous iodide 8mg (0.04mmol), tetrabutylammonium chloride 11mg (0.04mmol), lithium trifluoromethanesulfonate 31mg (0.2mmol ), nitrogen pumped for ventilation, then 3mL of 1,4-dioxane was added, and 20mg (0.2mmol) of phenylacetylene was added under stirring. React on a 60° C. electromagnetic heating stirrer for about 2 hours. After the reaction, use a 10mL sand core column filled with 3cm high silica gel to filter and elute with an appropriate amount of petroleum ether. The resulting filtrate is removed from the reaction solvent using a rotary evaporator, and purified by column chromatography to obtain 2-(4'-fluoro Phenyl)-4-phenyl 1,...
Embodiment 3
[0062] 2-(4’-Chlorophenyl)-4-phenyl 1,1-difluoro-1-en-3-yne
[0063] Take a 10mL Schlenk tube, put it into a magnet stopper and put a rubber stopper on it, heat and dry, cool under nitrogen, then add 1,1,1-trifluoromethyl-2-(4'-chlorophenyl)ketone and p-toluenesulfonate Acylhydrazone 75mg (0.2mmol), lithium tert-butoxide 32mg (0.4mmol), cuprous iodide 8mg (0.04mmol), tetrabutylammonium chloride 11mg (0.04mmol), lithium trifluoromethanesulfonate 31mg (0.2mmol ), nitrogen pumped for ventilation, then 3mL of 1,4-dioxane was added, and 20mg (0.2mmol) of phenylacetylene was added under stirring. React on a 60° C. electromagnetic heating stirrer for about 2 hours. After the reaction is over, use a 10mL sand core column with 3cm high silica gel to filter and elute with an appropriate amount of petroleum ether. The resulting filtrate is removed from the reaction solvent using a rotary evaporator, and purified by column chromatography to obtain 2-(4'-chloro Phenyl)-4-phenyl 1,1-diflu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com