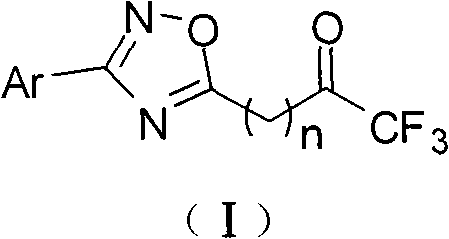
Trifluoromethyl ketone compound used as histone deacetylase inhibitor and application thereof
A compound and aryl technology, which is applied in the field of trifluoromethyl ketone histone deacetylase inhibitors, can solve the problems of high toxicity, insufficient curative effect, and few types of deacetylase inhibitors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Preparation of 1,1,1-trifluoro-8-(3-phenyl-1,2,4-oxadiazol-5-yl)-2-octanone (compound 1)
[0058]
[0059] Dissolve aniline oxime (5g, 36-7mmol) in 15mL pyridine, add 8-chloro-8-oxo-octanoic acid methyl ester (9.11g, 44.1mmol) dropwise within 30min at room temperature, and reflux reaction after addition , until the aniline oxime reaction was complete, cooled to room temperature, added ethyl acetate and water for distribution, the organic layer was washed successively with 2M aqueous hydrochloric acid solution, saturated sodium bicarbonate solution and saturated brine, the organic layer was dried with magnesium sulfate, passed through a silica gel column Chromatography gave a colorless oily liquid. Yield 65%. Heptanoic acid methyl esters of other substituents were synthesized in the same way, and the yield was 52-70%.
[0060] 1 H-NMR (500Hz, CDCl 3)δ: 8.06-8.08(m, 2H), 7.28-7.49(m, 3H), 3.66(s, CO2CH3, 3H), 2.94(t, J=7.5Hz, het-CH 2 CH 2 , 2H), 2.31(t...
Embodiment 2
[0064] Example 2 Preparation of 1,1,1-trifluoro-8-(3-p-tolyl-1,2,4-oxadiazol-5-yl)-2-octanone (compound 2)
[0065]
[0066] Preparation method: replace the aniline oxime in Example 1 with p-toluidine oxime, and the other preparation methods are the same.
[0067] Mp: 50-51°C. 1 H-NMR (300Hz, CDCl 3 )δ: 7.95(d, J=8.17Hz, 2H), 7.27(d, J=7.84Hz, 2H), 2.94(t, J=7.53Hz, het-CH 2 CH 2 , 2H), 2.72(t, J=7.19Hz, CH 2 CH 2 CO 2 CF 3 , 2H), 2.41(s, 3H), 1.86-1.93(m, CH 2 , 2H), 1.68-1.74 (m, CH 2 , 2H), 1.41-1.49(m, 2CH 2 , 4H). 13 C-NMR (75Hz, DMSO-d6) δ: 191.03 (q, J=34.2Hz), 179.52 (C-5), 168.22 (C-3), 141.39, 129.49, 127.27, 124.01, 115.53 (d, J= 290.48Hz), 36.13, 28.51, 28.16, 26.40, 26.26, 22.07, 21.26. HRMS [Found: m / z 339.1326 (M-H) - , 363.1291 (M+Na) + ;Calcd for C 17 h 19 f 3 N 2 o 2 : M, 340.1399.
Embodiment 3
[0068] Example 3 Preparation of 1,1,1-trifluoro-8-(3-p-methoxyphenyl-1,2,4-oxadiazol-5-yl)-2-octanone (compound 3)
[0069]
[0070] Preparation method: replace the aniline oxime in Example 1 with 4-methoxyanilinoxime, and the other preparation methods are the same.
[0071] Mp: 39-41°C. 1 H-NMR (300Hz, CDCl 3 )δ: 8.00(m, 2H), 6.98(m, 2H), 3.86(s, 3H), 2.93(t, J=7.53Hz, het-CH 2 CH 2 , 2H), 2.73(t, J=7.19Hz, CH 2 CH 2 CO 2 CF 3 , 2H), 1.83-1.93 (m, CH2, 2H), 1.66-1.75 (m, CH2, 2H), 1.36-1.45 (m, 2CH 2 , 4H). 13 C-NMR (75Hz, DMSO-d6) δ: 179.40 (C-5), 167.94 (C-3), 161.85, 128.95, 119.33, 114.20, 55.32, 36.15, 28.53, 28.18, 26.42, 26.28, 22.09. HRMS [Found: m / z 355.1275 (M-H) - , 379.1240 (M+Na) + ; Calcd for C 17 h 19 f 3 N 2 o 3 : M, 356.1348.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com