Method for preparing chiral beta-trifluoromethyl-beta-hydroxy-alpha-amino acid and derivatives thereof
A technology of trifluoromethyl and trifluoromethyl ketone, applied in the field of compound preparation, can solve the problems of few methods, many reaction steps, harsh conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
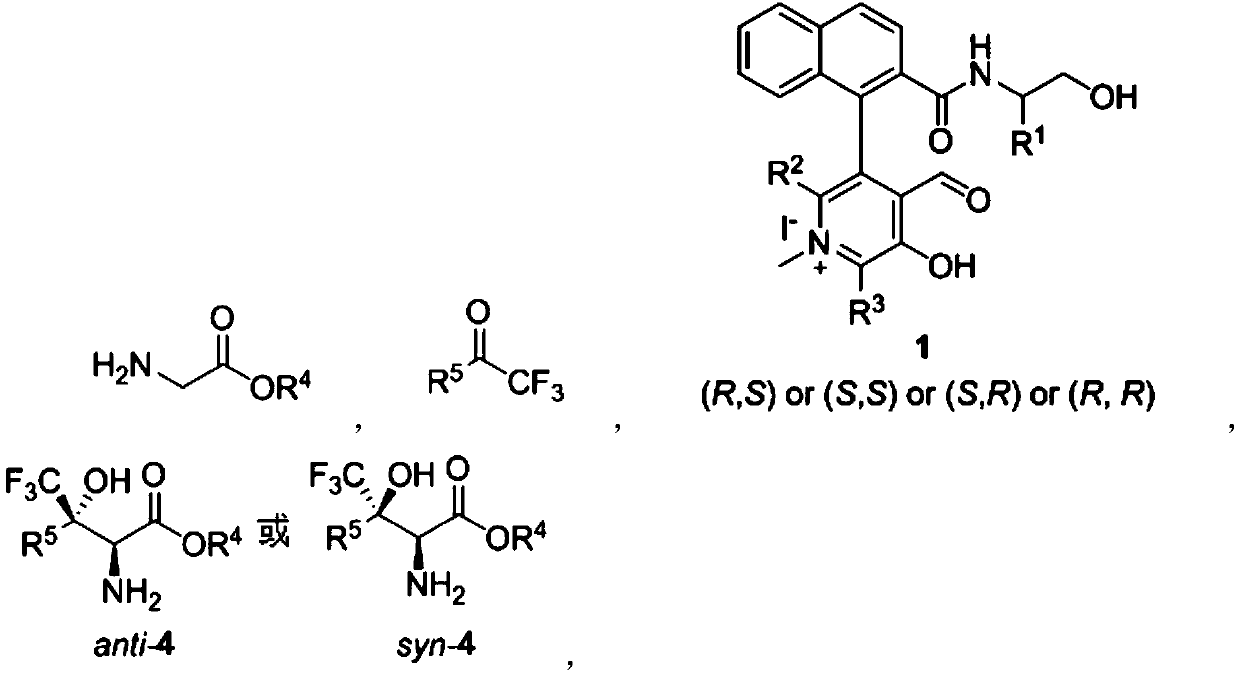
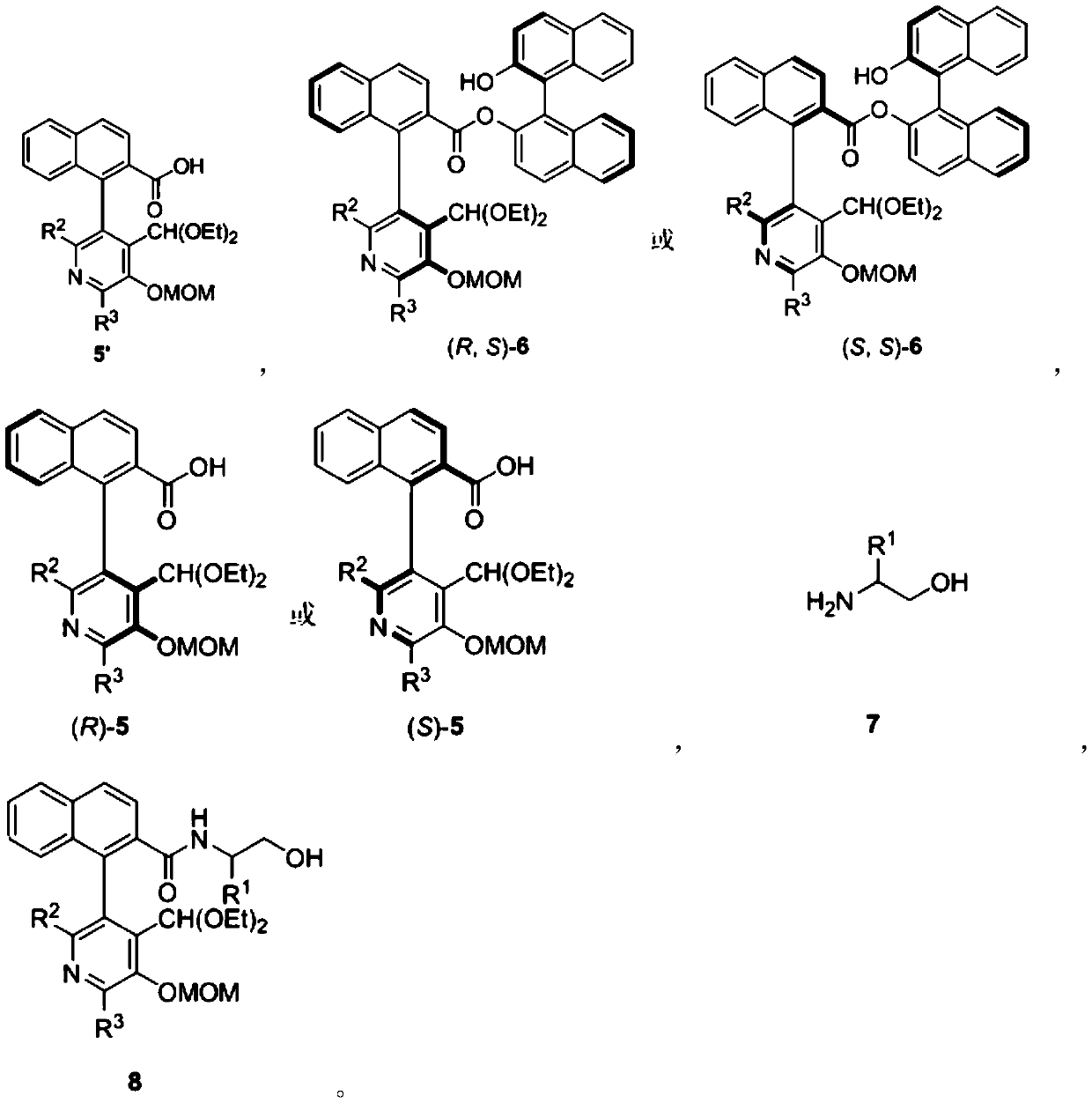
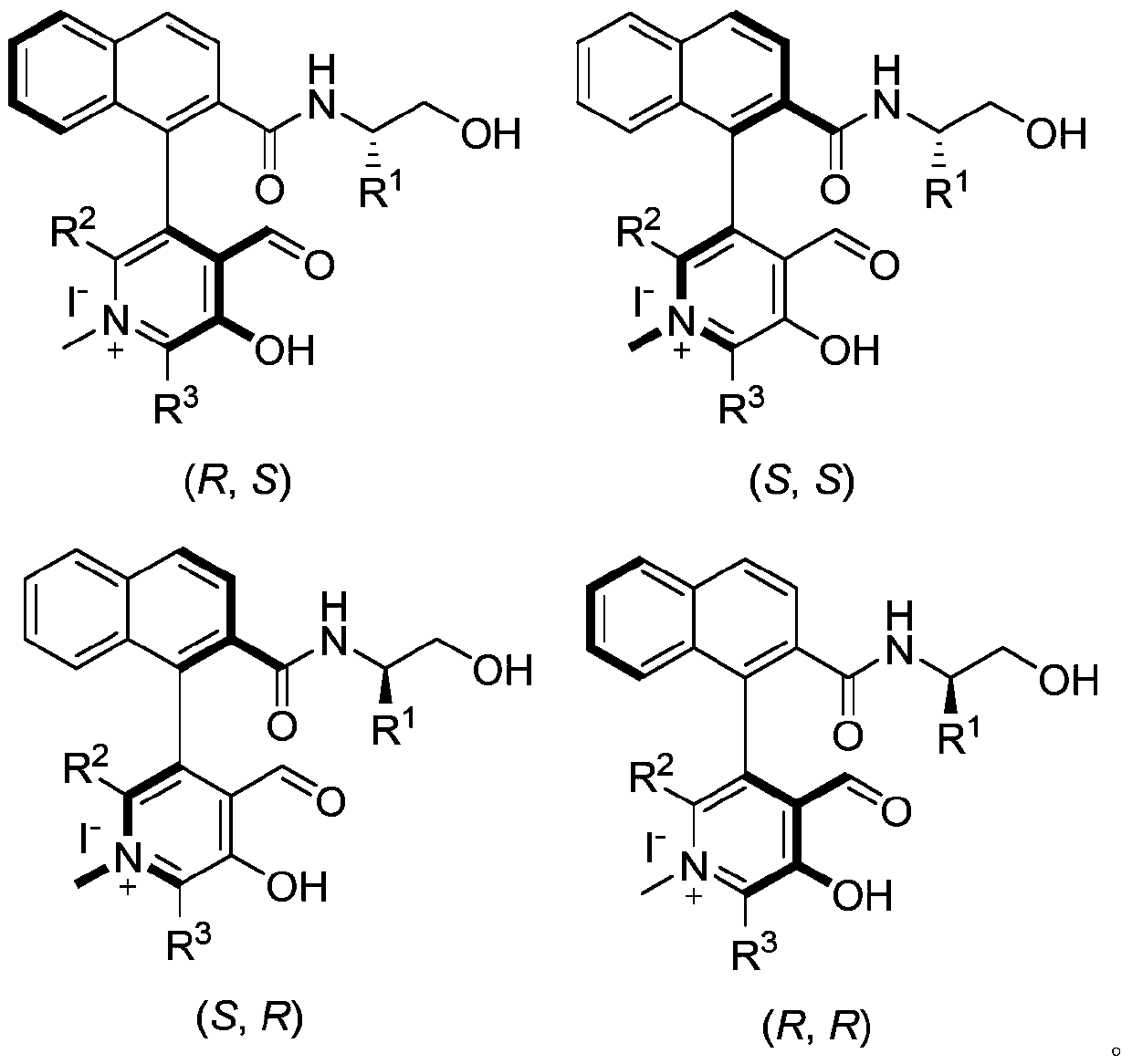
[0062] In the preparation process of the above-mentioned chiral pyridoxal catalyst, according to the needs of the obtained object product, R 1 , R 2 , R 3 Can be adjusted arbitrarily within the following ranges:
[0063] R 1 Can be hydrogen or C 1 -C 24 Hydrocarbyl, R 2 and R 3 independently hydrogen or C 1 -C 24 Hydrocarbyl; R 1 The chosen C 1 -C 24 Hydrocarbyl is methyl, ethyl, n-propyl, isopropyl, n-butyl, tert-butyl, cyclopentyl, cyclohexyl, cycloheptyl, phenyl, benzyl, (1-phenyl) ethyl, 1-naphthyl, 2-naphthyl or halogen, etc.; R 2 or R 3 The chosen C 1 -C 24 Hydrocarbyl is methyl, ethyl, n-propyl, isopropyl, n-butyl, tert-butyl, cyclopentyl, cyclohexyl, cycloheptyl, phenyl, benzyl, 2-biphenyl, 3-biphenyl , 4-biphenyl, 2,6-biphenyl, 3,5-biphenyl, 1-naphthyl or 2-naphthyl, etc.
[0064] In addition, the types and dosages of the corresponding reagents can also be adjusted as needed within the following ranges (i.e. choose any midpoint or end value):
[0065...
Embodiment 1
[0070] Synthesis of chiral β-trifluoromethyl-β-hydroxy-α-amino acid ester 4a catalyzed by chiral pyridoxal catalyst (S)-1
[0071]
[0072] Trifluoromethyl ketone 3a (0.252g, 1.0mmol), glycine tert-butyl ester 2 (0.195g, 1.50mmol), CH 3 COOH (0.030g, 0.50mmol) and chiral pyridoxal catalyst (S)-1 (0.0005g, 0.001mmol) were added to a 5mL reaction flask, a stirring bar was added, a rubber stopper was covered, and the N 2 Three times, inject CH 2 Cl 2 (4.0 mL), reacted at -10°C for 48 hours, added hydroxylamine hydrochloride (HONH 2 HCl, 0.070g, 1.0mmol) and sodium bicarbonate (NaHCO 3 , 0.084g, 1.0mmol), stirring at room temperature for 1 hour, spin off the organic solvent, column chromatography to obtain compound anti-4a (light yellow solid) and syn-4a (light yellow solid), (the total product weight 0.324g, total Yield 85%).
[0073] The dr value of 4a is obtained by 1 H NMR analysis of the reaction crude product, its dr value is 1:5; the ee value of anti-4a is obtained...
Embodiment 2
[0077] Synthesis of chiral β-trifluoromethyl-β-hydroxy-α-amino acid ester 4b catalyzed by chiral pyridoxal catalyst (S)-1
[0078]
[0079] Trifluoromethyl ketone 3b (0.278g, 1.0mmol), glycine tert-butyl ester 2 (0.195g, 1.50mmol), CH 3 COOH (0.030g, 0.50mmol) and chiral pyridoxal catalyst (S)-1 (0.0005g, 0.001mmol) were added to a 5mL reaction flask, a stirring bar was added, a rubber stopper was covered, and the N 2 Three times, inject CH 2 Cl 2 (4.0mL), reacted at -10°C for 48 hours, added hydroxylamine hydrochloride (HONH 2 HCl, 0.070g, 1.0mmol) and sodium bicarbonate (NaHCO 3 , 0.084g, 1.0mmol), stirring at room temperature for 1 hour, spin off the organic solvent, column chromatography to obtain compound anti-4b (light yellow solid) and syn-4b (light yellow solid), (the total product weight 0.372g, total Yield 91%).
[0080] The dr value of 4b is obtained by 1 H NMR analysis of the reaction crude product, its dr value is 1:6; the ee value of anti-4b is obtained ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com