Trifluoromethyl-substituted dihydropyridone derivatives, preparation method and application thereof
A technology of dihydropyridone and trifluoromethyl, which is applied in the field of synthesis of active compounds of agrochemical herbicides, and can solve the problems that the herbicidal activity of dihydropyridone derivatives has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
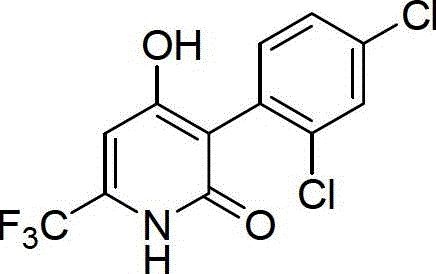
[0050] The preparation of embodiment 1 compound 04
[0051] (1) Preparation of 3-(2,4-dichlorophenyl)-6-trifluoromethyl-1,2,5,6-tetrahydro-2oxo-4-pyridylbenzyl carbonate
[0052] 0.200 g of 3-(2,4-dichlorophenyl)-6-trifluoromethyl-5,6-dihydro-4-hydroxypyridin-2(1H)-one and 0.062 g of triethylamine were dissolved in 10 mL of diethylamine In methyl chloride, 0.105 g of benzyl chloroformate was added dropwise, and reacted at room temperature for 1 hour. After the reaction, add 20 mL of 1mol / L dilute hydrochloric acid to wash for three times, then dry the organic layer with anhydrous sodium sulfate, filter, and remove the solvent to obtain 0.268 g of white solid, melting point: 164-165 °C. NMR data: 1 H NMR (400Hz, CDCl 3 ):δ7.42-7.37(m,4H),7.29-7.26(m,2H),7.19-7.13(m,1H),7.04-7.01(m,1H),6.18(d,1H),5.15(s ,2H), 4.28-4.11(m,1H), 3.36-3.22(m,1H), 3.05-2.95(m,1H).
[0053] (2) Preparation of 3-(2,4-dichlorophenyl)-6-trifluoromethyl-5,6-dihydro 4-hydroxypyridin-2(1H)-one
[0054] ...
Embodiment 2
[0071] The following is a test using the compounds provided by the present invention to verify the evaluation of the activity of barnyardgrass. It should be pointed out that the present invention is not limited to the scope of the following examples.
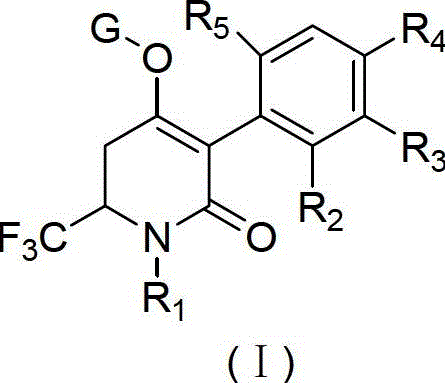
[0072] Dissolve the trifluoromethyl-substituted dihydropyridone derivatives represented by the structural formula (I) with serial numbers 1, 2, 3, 4, and 7 provided by the present invention in solvent, water, and surfactant respectively, and mix them to form Uniform water phase, which can be diluted with water to different concentrations of solutions during use. The test objects and test methods are as follows:
[0073] The barnyardgrass seeds to be tested were evenly sown into pots with an inner diameter of 9 cm, and cultivated in a greenhouse. When the barnyardgrass grows to the 2-2.5 leaf stage, the post-emergence stem and leaf treatment is carried out on the automatic spraying device. Each treatment was repeated 3 times, an...
Embodiment 3
[0077] Dissolve the trifluoromethyl-substituted dihydropyridone derivatives represented by the structural formula (I) with serial number 2 provided by the present invention in solvent, water and surfactant, mix to form a homogeneous water phase, and dilute with water when used To different concentrations of solutions, the test objects and test methods are as follows:
[0078] The Setaria seeds for testing were evenly sown into pots with an inner diameter of 9 cm, and cultivated in the greenhouse. After the foxtail grows to the 2-2.5 leaf stage, the post-emergence stem and leaf treatment is carried out on the automatic spraying device. Each treatment was repeated 3 times, and a blank control was set. After the treatment, it was left to stand for 4-5 hours. After the leaves were dried, they were moved into the greenhouse for cultivation. Observe the growth of the plants every day, regularly record the symptoms of damage, and visually investigate the comprehensive herbicidal act...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com