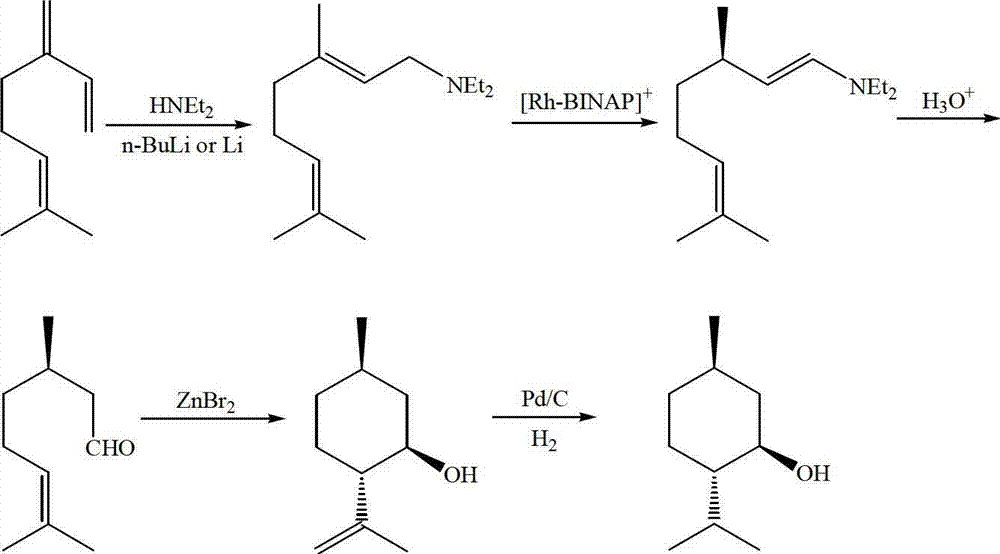
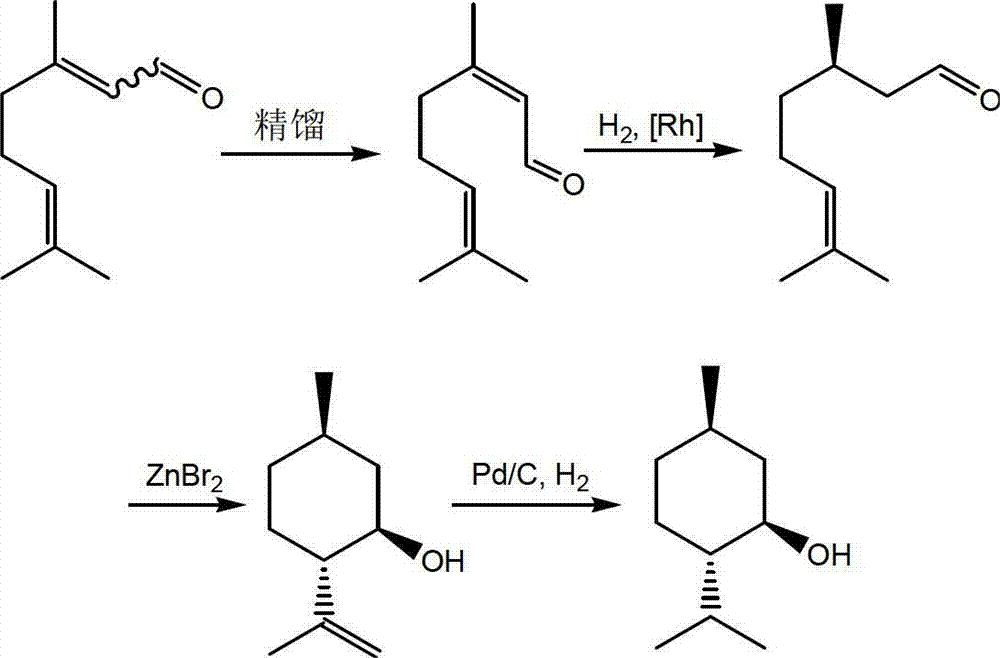
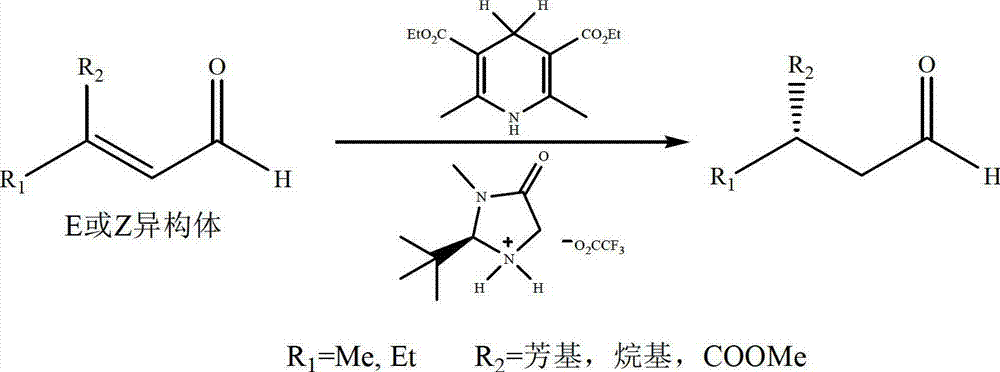
Method for asymmetric synthesis of levorotation menthol
A kind of L-menthol, asymmetric technology, applied in the field of three-step reaction synthesis of L-menthol, can solve the problems of trans-citral difficult to reuse, high production equipment requirements, etc., to achieve simple catalyst preparation, mild reaction conditions, and synthetic process simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Catalytic asymmetric hydrogenation
[0037] Take a 250ml three-necked flask, add citral (15.2g, 0.1mol), (R)-2-[bis(4-ethylphenyl)]methyltetrahydropyrrole (29.3mg , 0.1mmol), trifluoroacetic acid (12mg, 0.1mmol), 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate diethyl ester (75.9g, 0.3mol), and Add 80 mL of tetrahydrofuran to dissolve the solid. Stir at room temperature for 10 h, remove the solvent under reduced pressure, and purify by column chromatography to obtain 12.9 g of (R)-citronellal light yellow liquid with a yield of 84%.
[0038] 2. Ring closing reaction
[0039] 5°C, N 2 ZnBr 2 (1.35g, 6mmol) was added in portions to a stirred solution of (R)-citronellal (4.6g, 30mmol) in toluene (30mL), and the resulting reaction compound was stirred at 5-10°C for 0.5h. Filter, rinse ZnBr with n-hexane (10mL) 2 . The filtrate was successively washed with H 2 O (30mL), saturated NaHCO 3 solution (30mL) and saturated brine (30mL) for washing. anhydrous MgSO ...
Embodiment 2
[0043] 1. Catalytic asymmetric hydrogenation
[0044] Take a 250ml three-necked flask, add citral (15.2g, 0.1mol), (R)-2-[bis(4-ethylphenyl)]methyltetrahydropyrrole (29.3mg , 0.1mmol), trifluoroacetic acid (12mg, 0.1mmol), 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate diethyl ester (75.9g, 0.3mol), and Add 80ml of tetrahydrofuran to dissolve the solid, stir at -20°C for 20h, remove the solvent under reduced pressure, and purify by column chromatography to obtain 8.7g of (R)-citronellal light yellow liquid with a yield of 71%.
[0045] 2. Ring closing reaction
[0046] 5°C, N 2 ZnBr 2 (1.35g, 6mmol) was added in portions to a stirred solution of (R)-citronellal (4.6g, 30mmol) in toluene (30mL), and the resulting reaction compound was stirred at 5-10°C for 0.5h. Filter, rinse ZnBr with n-hexane (10mL) 2 . The filtrate was successively washed with H 2 O (30mL), saturated NaHCO 3 solution (30mL) and saturated brine (30mL) for washing. anhydrous MgSO 4 After drying, th...
Embodiment 3
[0050] 1. Catalytic asymmetric hydrogenation
[0051] Take a 250ml three-necked flask, add citral (15.2g, 0.1mol), (R)-2-[bis(4-ethylphenyl)]methyltetrahydropyrrole (29.3mg , 0.1mmol), trifluoroacetic acid (12mg, 0.1mmol), 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate diethyl ester (75.9g, 0.3mol), and Add 80 mL of tetrahydrofuran to dissolve the solid. Stir at 40°C for 10 h, remove the solvent under reduced pressure, and purify by column chromatography to obtain 10.3 g of (R)-citronellal light yellow liquid with a yield of 74%.
[0052] 2. Ring closing reaction
[0053] 5°C, N 2 ZnBr 2 (1.35g, 6mmol) was added in portions to a stirred solution of (R)-citronellal (4.6g, 30mmol) in toluene (30mL), and the resulting reaction compound was stirred at 5-10°C for 0.5h. Filter, rinse ZnBr with n-hexane (10mL) 2 . The filtrate was successively washed with H 2 O (30mL), saturated NaHCO 3 solution (30mL) and saturated brine (30mL) for washing. anhydrous MgSO 4 After dryi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com