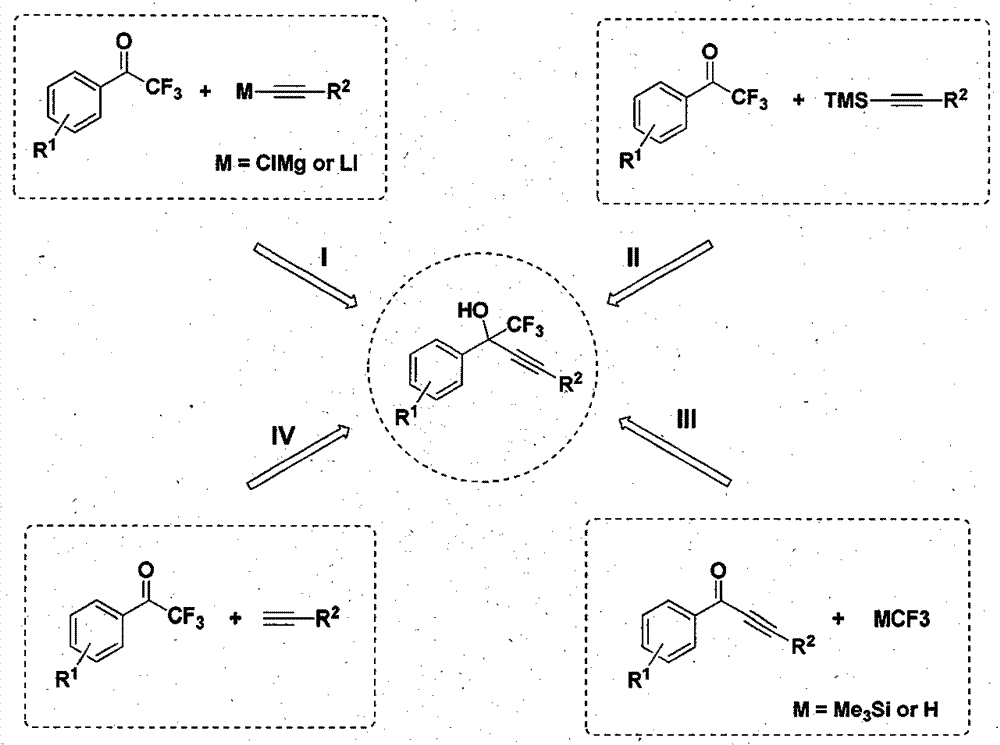
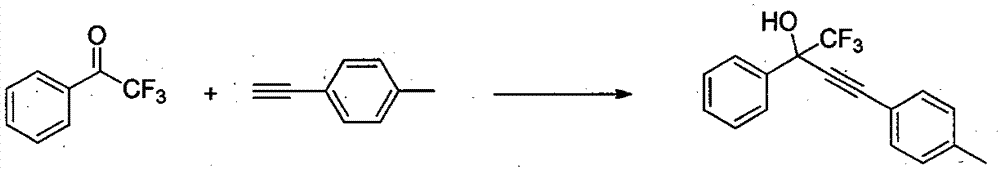A simple and efficient method for preparing efavirenz intermediates
A technology of efavirenz and intermediates, which is applied in the field of organic compound catalytic chemistry, can solve the problems of low atom economy, high cost, and high cost, and achieve the effect of wide application prospects and simple and efficient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0014]
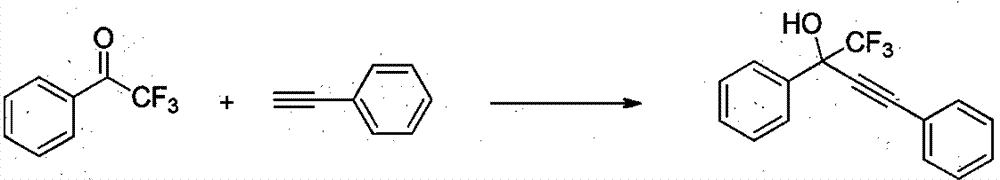
[0015] 2,2,2-trifluoroacetophenone (0.5mmol), phenylacetylene (1.0mmol), CuI (0.05mmol), K 2 CO 3 (0.1mmol) and THF (1mL) were added into a Schlinke bottle, stirred and reacted at 50°C for 24h, followed by TLC. After the reaction was completed, 15 mL of saturated brine was added to quench the reaction, the reaction mixture was extracted with dichloromethane (15 mL × 3) to extract the reaction product, the organic phases were combined, concentrated using a rotary evaporator to obtain a crude product, and the target product was obtained by column chromatography. The eluent used for analysis was petroleum ether: ethyl acetate (10:1), and the structure of the product was identified by NMR and high-resolution mass spectrometry. The separation yield reaches 99%.
specific Embodiment 2
[0016]
[0017] 2,2,2-trifluoroacetophenone (0.5mmol), 4-methylphenylacetylene (1.0mmol), CuCl (0.05mmol), Na 2 CO 3 (0.1mmol) and DMF (1mL) were added into a Schlinke bottle, stirred and reacted at 50°C for 24h, followed by TLC. After the reaction was completed, 15 mL of saturated brine was added to quench the reaction, the reaction mixture was extracted with dichloromethane (15 mL × 3) to extract the reaction product, the organic phases were combined, concentrated using a rotary evaporator to obtain a crude product, and the target product was obtained by column chromatography. The eluent used for analysis was petroleum ether: ethyl acetate (10:1), and the structure of the product was identified by NMR and high-resolution mass spectrometry. The separation yield reaches 94%.
specific Embodiment 3
[0018]
[0019] 2,2,2-trifluoroacetophenone (0.5mmol), 4-methoxyphenylacetylene (1.0mmol), Cu (0.05mmol), NaHCO 3 (0.1mmol) and toluene (1mL) were added into a Schlinke bottle, stirred and reacted at 50°C for 24h, followed by TLC. After the reaction was completed, 15 mL of saturated brine was added to quench the reaction, the reaction mixture was extracted with dichloromethane (15 mL × 3) to extract the reaction product, the organic phases were combined, concentrated using a rotary evaporator to obtain a crude product, and the target product was obtained by column chromatography. The eluent used for analysis was petroleum ether: ethyl acetate (10:1), and the structure of the product was identified by NMR and high-resolution mass spectrometry. The separation yield reaches 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com