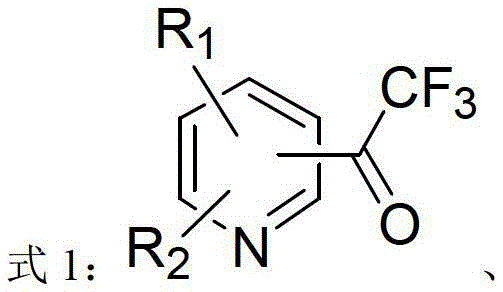
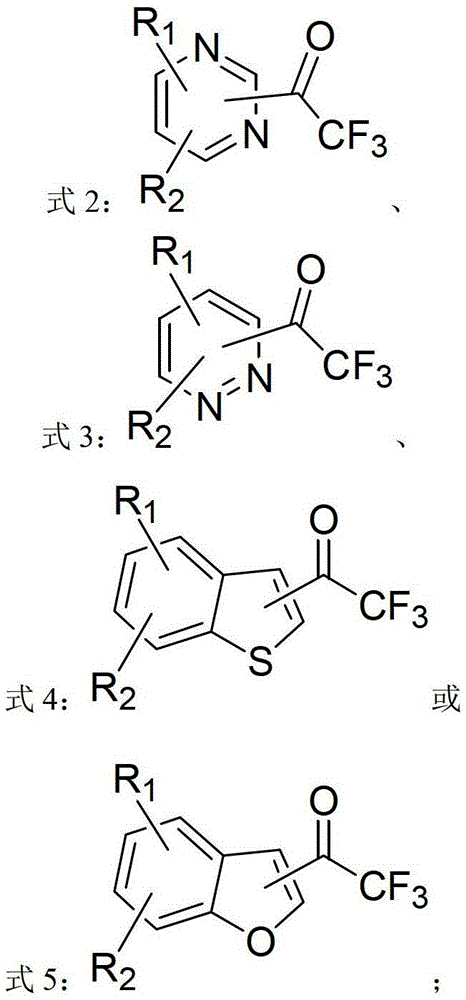
Heterocycle-containing trifluoromethyl ketone compound and preparation method thereof
A technology for trifluoromethyl ketones and heterocyclic compounds, which is applied in the field of heterocyclic-containing trifluoromethyl ketone compounds and its preparation, can solve problems such as no further research and no preparation of trifluoromethyl ketone compounds, and achieve Wide applicability, low cost, and high productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
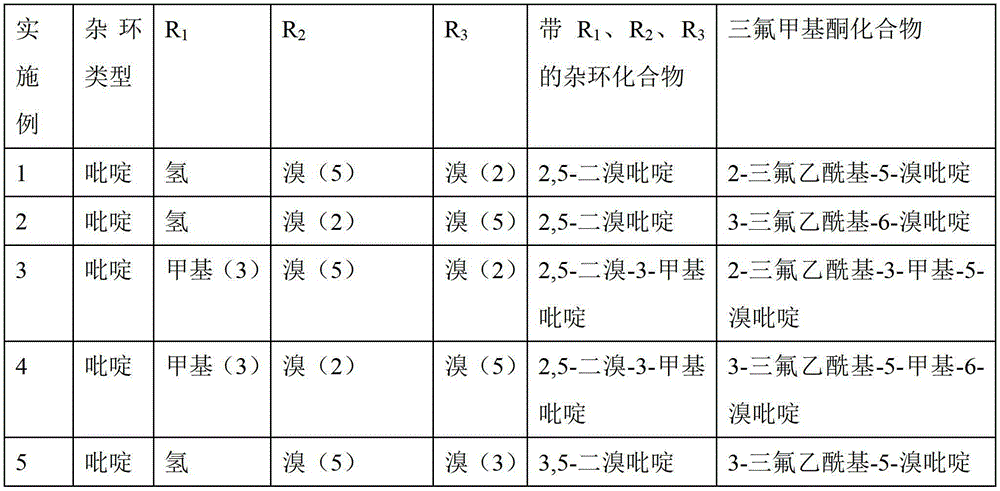
[0029] The heterocyclic-containing trifluoromethyl ketone compound in this example is 2-trifluoroacetyl-5-bromopyridine. with R 1 , R 2 , R 3 The heterocyclic compound is 2,5-dibromopyridine, where R 1 for hydrogen, R 2 is bromine(5), R 3 is bromo(2), and the heterocycle type of the heterocyclic compound is pyridine.
[0030] The preparation method of the heterocyclic-containing trifluoromethyl ketone compound of the present embodiment comprises the following steps:
[0031] 1) Dissolve 10mmol of 2,5-dibromopyridine in 20ml of toluene, slowly add organolithium reagent (n-BuLi, 11mmol) dropwise under nitrogen protection, and react at -78°C for 2h to obtain organolithium intermediate body;
[0032] 2) Slowly add 15 mmol of N-trifluoroacetylmorpholine dropwise to the organolithium intermediate obtained in step 1), react at -78°C for 2 hours, raise the temperature to room temperature naturally, stir overnight, and then use at 20°C 10ml of water is used as a quenching agent...
experiment example
[0054] This experimental example detected the product yield of the preparation method of the trifluoromethyl ketone compound of Examples 1-26, and carried out NMR characterization to the product obtained by the preparation method of the trifluoromethyl ketone compound of Examples 1-26, the result As shown in Table 5.
[0055] Table 5 Product yield and NMR characterization of the preparation methods of trifluoromethyl ketone compounds in Examples 1-26.
[0056]
[0057]
[0058]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com