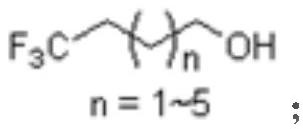Novel synthesis method of 4, 4, 4-trifluoro-1-butanol and homologue thereof
A homologous, newly synthesized technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of carbon-based compounds, etc., can solve the problems of low yield, high activity of tetrahydroaluminum lithium, and incompatibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
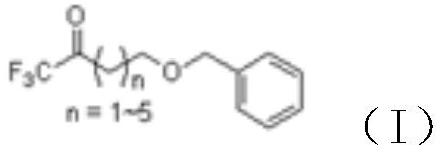
[0033] (1) Preparation of formula (I) compound (2-benzyloxyethyl)-trifluoromethyl ketone
[0034]
[0035] Add 700mL of 2-benzyloxy-ethylmagnesium chloride (1M) dropwise to 1000mL of ethyl trifluoroacetate at -20°C, slowly return to room temperature, react for 6 hours, pour into 1000mL of water, adjust the pH to 5.0-6.8 with hydrochloric acid , collected the organic phase, and spin-dried the solvent to obtain (2-benzyloxyethyl)-trifluoromethyl ketone. The crude product was temporarily overweight and was directly used in the next reaction.
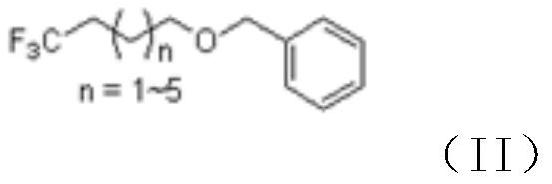
[0036] (2) Preparation of formula (II) compound 1-benzyloxy-4,4,4-trifluorobutane
[0037]
[0038] Dissolve (2-benzyloxyethyl)-trifluoromethyl ketone obtained in the previous step in 900mL DMSO, add 50g hydrazine hydrate, react at room temperature for 2 hours, slowly add 200g potassium tert-butoxide in batches, continue to Reacted at room temperature for 12 hours, poured into ice water, adjusted to neutral with hydrochloric acid, ex...
Embodiment 2
[0043] The preparation process is substantially the same as in Example 1, except that:
[0044] In step (3), the organic solvent is 1,2-dichloroethane, the Lewis acid is aluminum trichloride, and the three-step total reaction yield is 75%.
Embodiment 3
[0046] The preparation process is substantially the same as in Example 1, except that:
[0047] In step (3), the organic solvent is chloroform, the Lewis acid is hydrobromic acid, and the three-step total reaction yield is 69%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com