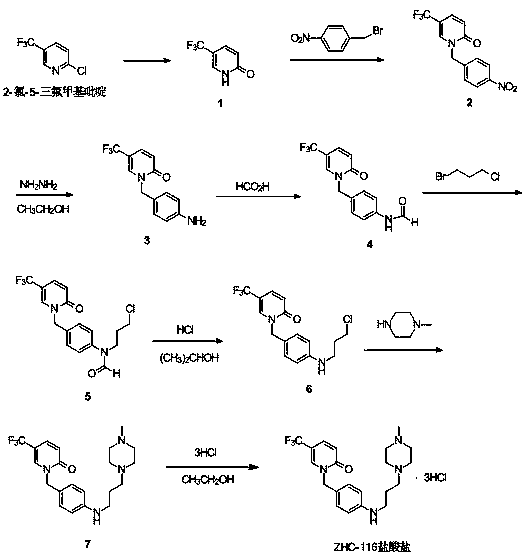
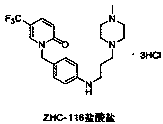
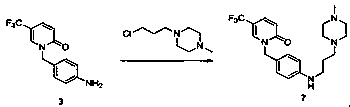
Synthetic method of renal fibrosis resistant medicine 1-(substituted benzyl)-5- trifluoromethyl-2(1H)-pyridone hydrochloride
A technology of trifluoromethylpyridine and trifluoromethyl, applied in the field of ZHC-116 hydrochloride), can solve the problems of ring-forming side reactions, affecting product yield, low reported yield, etc. The effect of complete reaction and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Preparation of 5-trifluoromethylpyridin-2(1H)-one (compound 1)
[0041] In the reaction bottle, add 2-chloro-5-trifluoromethylpyridine 50g (275mmol), dioxane 60mL, 8M hydrochloric acid 60mL (480mmol), stir and heat to 90-94 0 C, react for 17-19 hours, until the TLC plate shows that the reaction is complete, and the temperature is controlled at 70 0 C. Evaporate the solvent under reduced pressure until no obvious liquid distills out, add 60mL of water, stir for 20min, filter with suction, wash the filter cake with 200mL of water until neutral, and 0 C was dried in vacuo to obtain 40.0 g of a yellow solid (compound 1), with a yield of 88%. 1 H NMR (500 MHz, DMSO-d6) δ (ppm): 6.68 (dd, 1H, J = 9.6Hz), 7.63(dd, 1H, J 1 = 9.6Hz, J 2 = 2.6Hz), 7.78(s, 1H), 13.15(s, 1H). 13 C NMR (125 MHz, DMSO-d6) δ (ppm): 111.19, 121.13, 123.18, 134.29, 137.57. MS(ESI) m / z: 164.0 [M+H] + . The product test data is consistent with the structure.
Embodiment 2
[0042] Example 2 1-(4-nitrobenzyl)-5-trifluoromethylpyridin-2(1H)-one (compound 2)
[0043] In the reaction flask, add compound 1 40g (245mmol), p-nitrobenzyl bromide 79.3g (368mmol), dioxane 300mL, K 2 CO 3 64.0g (463mmol), stirred and heated to 105 0 C was refluxed, and after 2 hours of reaction, it was heated and filtered, and the filter residue was washed with dioxane, and the combined filtrate was heated at 60 0 C reduced pressure to evaporate the solvent, temperature control 78-80 0 C. Add 95% ethanol dropwise to the concentrate until the solution is dissolved, stir for 30min, and cool to 0 0 C, suction filtration, wet product at 60 0 C was dried in vacuo to obtain 58.0 g of light yellow solid (compound 2), with a yield of 90%. 1 H NMR (500 MHz, DMSO-d6) δ (ppm): 5.25 (s, 2H), 6.71 (d, J = 9.6Hz, 1H), 7.47(d, J 1 = 9.4Hz, J 2 = 2.6 Hz 1H), 7.48(d, J = 8.7 Hz, 2H), 7.55(s,1H), 8.23(d, J = 8.8 Hz, 2H). 13 C NMR (125 MHz, DMSO-d6) δ (ppm): 52.38, 110.39, ...
Embodiment 3
[0044] Example 3 1-(4-aminobenzyl)-5-trifluoromethylpyridin-2(1H)-one (compound 3)
[0045] In the reaction bottle, add compound 2 50.0g (168mmol), 95% ethanol 300mL, stir and heat to 74 0 C, slowly drop in 33.6g (672mmol) of 80% hydrazine hydrate, after adding, heat to 75-80 0 C stirred overnight, concentrated the reaction solution to 1 / 4 under reduced pressure, added ethyl acetate (200mL)-water (100mL) mixture, stirred and separated, the aqueous phase was washed with 50mL of ethyl acetate, combined organic phase, washed with 50mL of brine Once, the organic phase was concentrated to dryness under reduced pressure, 50 mL of n-heptane was added, stirred for 30 min, and filtered to obtain 43.9 g of white solid (compound 3), with a yield of 96%. 1 H NMR (500 MHz,DMSO-d6) δ(ppm): 3.75 (s, 2H), 5.03(s, 2H), 6.66 (d, J = 6.7 Hz, 1H), 6.68 (d, J = 8.4 Hz, 2H), 7.15 (d, J = 8.3Hz, 2H), 7.42 (dd, J 1 = 9.6, J 2 = 2.6 Hz, 1H), 7.64 (s, 1H). 13 C NMR (125 MHz, DMSO-d6) δ (p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com