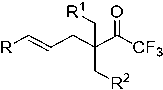
Alpha-quaternary carbon trifluoromethyl ketone compound and preparation method thereof
A technology of quaternary carbon trifluoromethyl ketone and trifluoromethyl ketone is applied in the field of α-quaternary carbon trifluoromethyl ketone compound and its preparation, and can solve problems such as high price and excess
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] Example 1 Preparation of 2-(p-methylcinnamyl)-2-trifluoroacetyl-3,4-dihydro-1-naphthalenone (A)
[0025]
[0026] Under argon protection, add Pd (PPh 3 ) 4 (0.005 mmol), dried solvent (1 mL), then add 2-trifluoroacetyl-3,4-dihydro-1-naphthone (0.11 mmol,), p-methylcinnamyl carbonate (1 mmol), TLC monitoring, column chromatography purification after the reaction to obtain the target product, yield 98%. 1 H NMR (600 MHz, CDCl 3 ) δ 8.07 (dd, J = 7.8, 0.7 Hz, 1H), 7.55 (td, J = 7.5, 1.3 Hz, 1H), 7.36(t, J = 7.6 Hz, 1H), 7.27 (d, J = 7.7 Hz, 1H), 7.22 (d, J = 8.0 Hz, 2H), 7.10(d, J= 7.9 Hz, 2H), 6.48 (d, J = 15.8 Hz, 1H), 6.20 – 6.02 (m, 1H), 3.22 –3.08 (m, 1H), 2.98 (ddd, J = 14.8, 11.5, 6.0 Hz, 2H), 2.74 (dd, J = 14.3, 8.6Hz, 2H), 2.32 (s, 3H), 2.24 (dt, J = 13.6, 4.8 Hz, 1H). 13 C NMR (151 MHz, CDCl 3 ) δ 194.24, 190.48 (q, J = 33.8 Hz), 142.78, 137.85, 135.02, 134.73,134.16, 130.70, 129.52, 129.16, 128.52, 127.58, 126.47, 122.59, 115.78 (q, ...
example 2
[0027] Example 2 Preparation of (S)-2-(p-methylcinnamyl)-2-trifluoroacetyl-3,4-dihydro-1-naphthalenone (B)
[0028]
[0029] Under argon protection, add Pd in a 10 mL catalytic test tube 2 (dba) 3 (0.005 mmol), chiral ligand (0.012 mmol) dried solvent (1 mL), at 30 o C stirred for half an hour, then added 2-trifluoroacetyl-3,4-dihydro-1-naphthone (0.11 mmol), p-methylcinnamyl carbonate (1 mmol), monitored by TLC, after the reaction, the column Purification by chromatography gave the target product in a yield of 96% and an enantiomeric excess of 94%. 1 H NMR (600 MHz, CDCl 3 ) δ 8.07 (dd, J = 7.8,0.7 Hz, 1H), 7.55 (td, J = 7.5, 1.3 Hz, 1H), 7.36 (t, J = 7.6 Hz, 1H), 7.27(d, J = 7.7 Hz, 1H), 7.22 (d, J = 8.0 Hz, 2H), 7.10 (d, J = 7.9 Hz, 2H), 6.48(d, J = 15.8 Hz, 1H), 6.20 – 6.02 (m, 1H), 3.22 – 3.08 (m, 1H), 2.98 (ddd, J = 14.8, 11.5, 6.0 Hz, 2H), 2.74 (dd, J = 14.3, 8.6 Hz, 2H), 2.32 (s, 3H), 2.24 (dt, J = 13.6, 4.8 Hz, 1H). 13 C NMR (151 MHz, CDCl ...
example 3
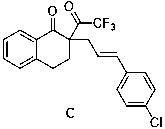
[0030] Example 3 Preparation of 2-p-chlorocinnamyl-2-trifluoroacetyl-3,4-dihydro-1-naphthalenone (C)
[0031]
[0032] Under argon protection, add Pd (PPh 3 ) 4 (0.005 mmol), dried solvent (1 mL), then added 2-trifluoroacetyl-3,4-dihydro-1-naphthone (0.11 mmol,), p-chlorocinnamyl carbonate (1 mmol), TLC After monitoring, after the reaction was completed, the target product was obtained by column chromatography purification with a yield of 95%. 1 H NMR (600 MHz, CDCl 3 ) δ 8.08 (d, J = 7.3 Hz, 1H), 7.56 (td, J = 7.6, 1.2 Hz, 1H), 7.46 (s,1H), 7.39 (d, J = 7.6 Hz, 1H), 7.37 – 7.34 (m, 1H), 7.29 (s, 1H), 7.26 (s,2H), 7.24 (d, J = 7.8 Hz, 1H), 7.16 (t, J = 7.8 Hz, 1H), 6.44 (d, J = 15.8Hz, 1H), 6.25 – 6.11 (m, 1H), 3.22 – 3.09 (m, 1H), 3.01 (ddd, J = 15.1, 10.8,5.7 Hz, 3H), 2.85 – 2.70 (m, 2H), 2.22 (dt, J = 13.6, 4.9 Hz, 2H). 13 C NMR (151MHz, CDCl 3 ) δ 194.01, 190.42 (q,J=33.8 Hz), 142.64, 139.07, 134.85, 133.76,130.86, 130.71, 130.34, 129.5, 129.19, 128....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com