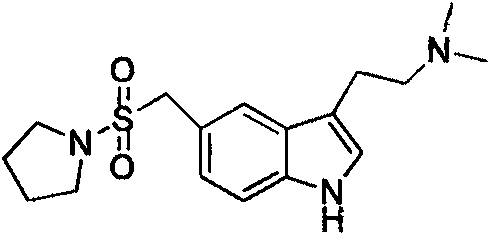
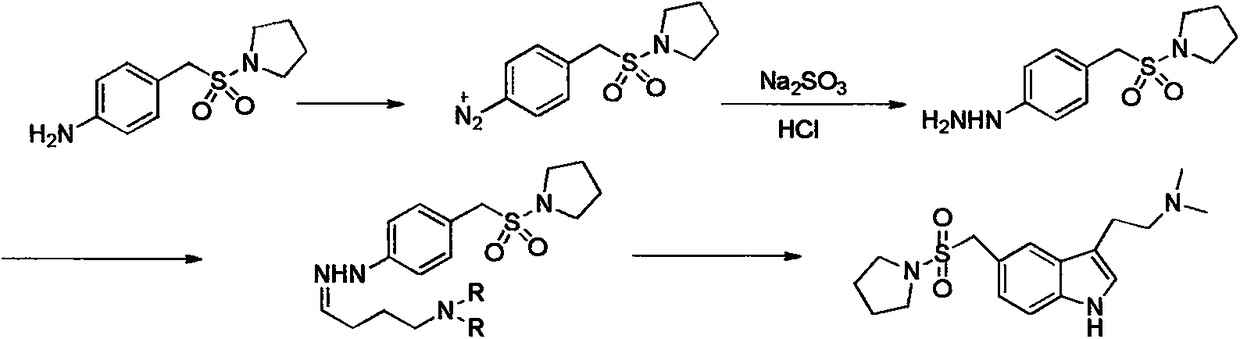
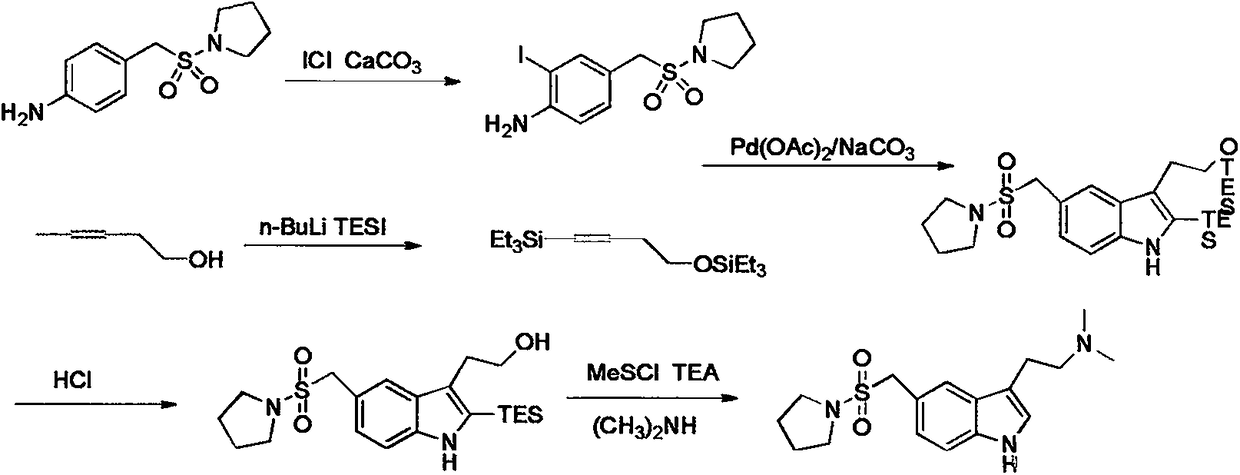
Preparation method of almotriptan key intermediate 1-(4-amino-phenylmethylsulfonyl)pyrrolidine
A technology of phenylmethylsulfonyl and almotriptan is applied in the field of preparation of key intermediate 1-pyrrolidine, and can solve the problems of difficult post-processing, unsuitable large-scale industrialization and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] A kind of preparation method of key intermediate 1-(4-amino-phenylmethylsulfonyl) pyrrolidine of almotriptan, its main steps are as follows:
[0026] Step 1: Prepare the Vilsmeier reagent with triphosgene (BTC) and N-dimethylformamide (DMF) for subsequent use, p-nitrobenzene methanesulfonic acid is a raw material, and react with Vilsmeier reagent sulfonylation under alkali-catalyzed conditions, and use TLC Monitor the reaction process, generate p-nitrobenzene methanesulfonyl chloride (formula 1), directly carry out the next step reaction without processing;
[0027]
[0028] Step 2: Using the p-nitrobenzenesulfonyl chloride obtained in Step 1 as a raw material, carry out a nucleophilic addition-elimination reaction with pyrrole under alkali catalysis, monitor the reaction with TLC, and extract it with saturated sodium bicarbonate and saturated saline after the reaction , the organic phase was dried with anhydrous sodium sulfate and distilled under reduced pressure to...
Embodiment 1
[0043] Step 1: Preparation of p-nitrobenzenesulfonyl chloride:
[0044] Take 7.3g of DMF (0.1mol) in an ice bath and cool down to below 0°C, stir with a magnetic force, dissolve 9.86g of Sanguangchrome BTC (0.033mol) in 10ml of anhydrous dichloromethane, slowly drop into DMF, and control the reaction at 0°C Below, stir and react for 30 minutes after the dropwise addition is completed, and the Vilsmeier reagent is seen as a white solid; Reaction, TLC detection (development phase: ethyl acetate:petroleum ether=2:1) After reacting for 2h, the system was cooled and concentrated to 10ml for later use;
[0045] Step 2: Preparation of 1-(4-nitro-phenylmethylsulfonyl)pyrrolidine:
[0046] Take 10ml of the p-nitrobenzenemethanesulfonyl chloride system in step 1, add 10.1g (0.1mol) of triethylamine, dissolve 6.7g (0.1mol) of pyrrole in 10ml of dichloromethane, drop into the p-nitrobenzenemethanesulfonyl chloride system, After 30 minutes of dropwise addition, keep the reaction at 60°C ...
Embodiment 2
[0050] Step 1: the preparation of p-nitrobenzene methanesulfonyl chloride:
[0051] Take 7.3g of DMF (0.1mol) and cool it down to below 0°C in an ice bath, stir it magnetically, dissolve 9.86g of BTC (0.033mol) in 10ml of anhydrous tetrahydrofuran, slowly drop it into DMF, control the reaction below 0°C, and complete the dropwise addition After stirring the reaction for 30min. 21.6g of p-nitrobenzenemethanesulfonic acid (0.1mol) was dissolved in 10ml of anhydrous tetrahydrofuran, slowly dropped into the Vilsmeier reagent system, reacted at 40°C, and detected by TLC (developing phase: ethyl acetate:petroleum ether=2:1) After 3.5 hours, the system was cooled and concentrated to 10ml for later use;
[0052] Step 2: Preparation of 1-(4-nitro-phenylmethylsulfonyl)pyrrolidine
[0053] Take 10ml of the p-nitrobenzenemethanesulfonyl chloride system in step 1, add 7.91g (0.1mol) of pyridine, dissolve 6.7g (0.1mol) of pyrrole in 10ml of dichloromethane, drop into the p-nitrobenzenemet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com