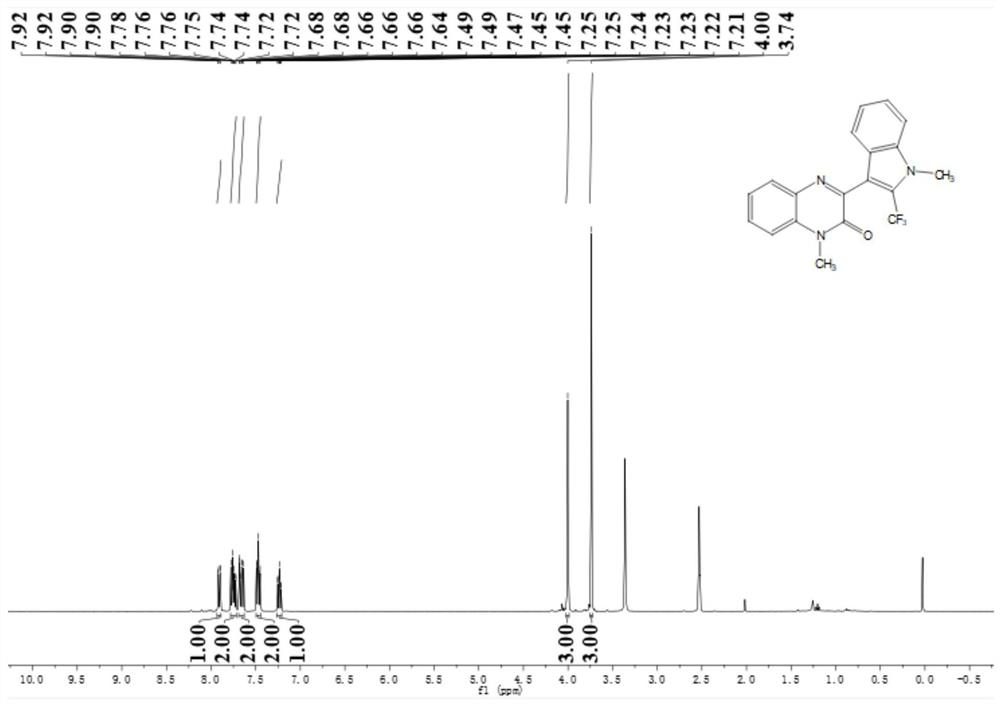
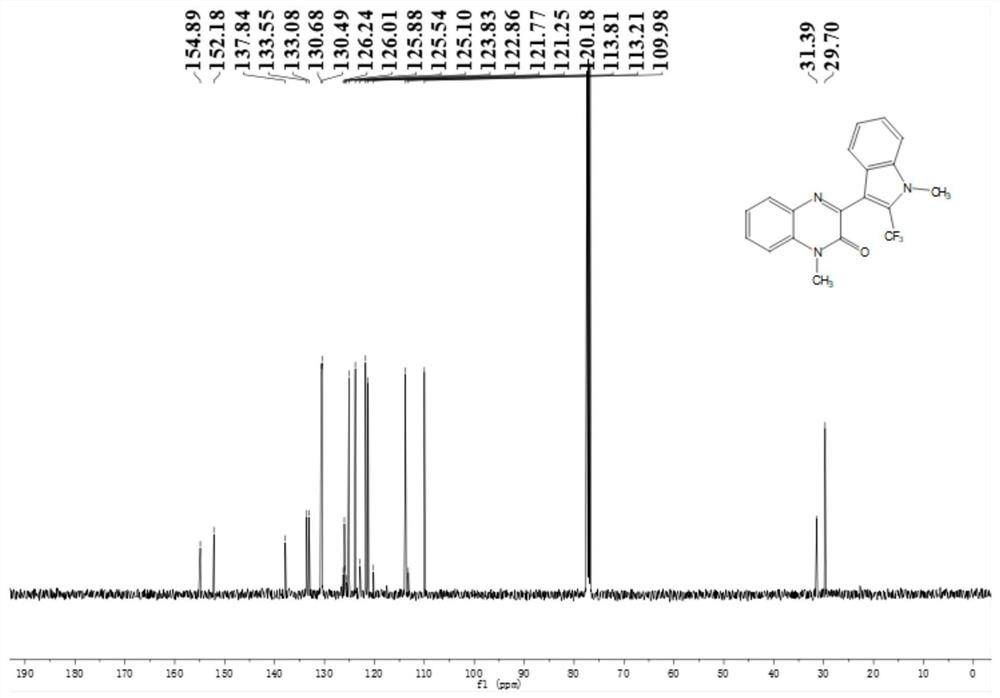
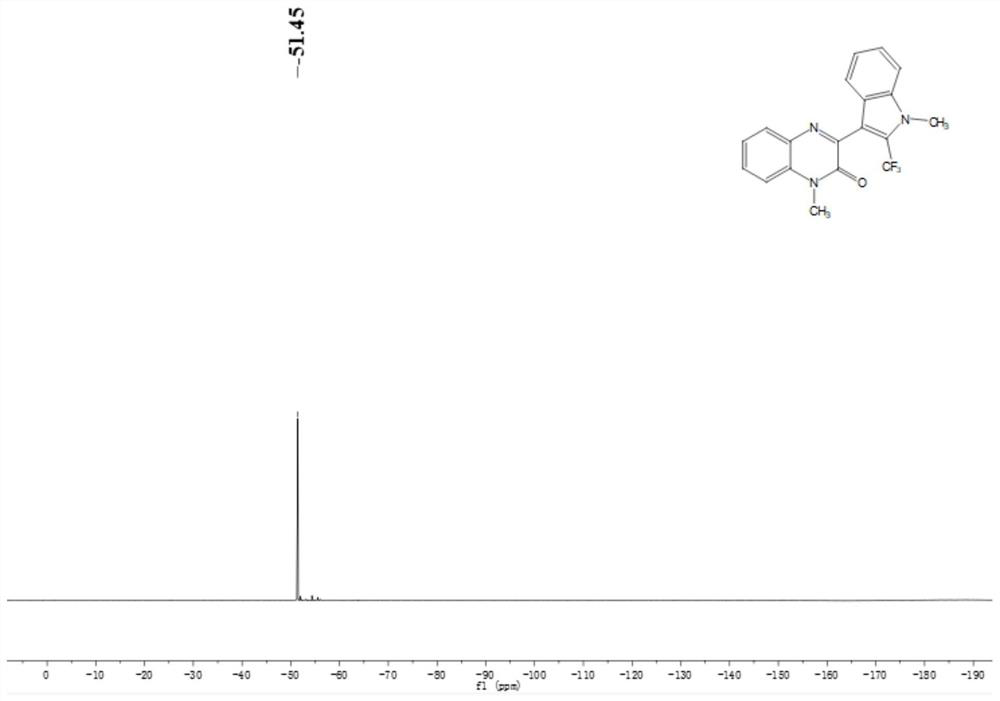
Application of solid acid catalysis multi-component reaction in preparation of fluorine-containing medicine
A multi-component reaction, solid acid catalysis technology, applied in the fields of drug combination, organic chemistry, anti-tumor drugs, etc., can solve the problems of metal catalysts that cannot be recycled, toxic solvents, etc., and achieve the effect of promoting industrialization and high-efficiency recycling ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Preparation of N-methylquinoxalinone (R in structural formula (II) 1 is methyl, R 2 for H)
[0061] 2-Hydroxyquinoxalinone (12mmol, 1.75g), potassium carbonate (24mmol, 3.31g) and 15mL N,N-dimethylformamide (DMF) were added to a 100mL round-bottomed flask, and the mixture was stirred in an ice bath. Then, the DMF solution of methyl iodide (14.4 mmol, 2 g) was added dropwise to the round-bottomed flask, and the reaction was continued at room temperature for 6 hours after the dropwise addition, and the reaction was detected by TLC. After the reaction was completed, the reaction solution was washed with saturated ammonium chloride solution, extracted with ethyl acetate, the organic phase was washed with brine, the organic phase was separated, and spin-dried to obtain a crude product, which was then washed with ethyl acetate / petroleum ether (1 : 4) Carry out recrystallization to obtain purer N-methylquinoxalinone.
[0062] Preparation of N-methyl indole (R in structural ...
Embodiment 2
[0069] The effect of different catalysts
[0070] The cation exchange resins Amberlst-21 and Amberlite-732 were used to replace Amberlyst-15, respectively, and the yields of the obtained target products were 64% and 79%, respectively.
Embodiment 3
[0072] Preparation of N-propargyl quinoxalinone (R in structural formula (II) 1 is propargyl, R 2 for H)
[0073] 2-Hydroxyquinoxalinone (12mmol, 1.75g), potassium carbonate (24mmol, 3.31g) and 15mL N,N-dimethylformamide (DMF) were added to a 100mL round-bottomed flask, and the mixture was stirred in an ice bath. , and then dropwise added the DMF solution of 3-bromopropyne (14.4 mmol, 1.71 g) into the round-bottomed flask. After the dropwise addition, the reaction was continued at room temperature for 6 hours, and the reaction was detected by TLC. After the reaction was completed, the reaction solution was washed with saturated ammonium chloride solution, extracted with ethyl acetate, the organic phase was washed with brine, the organic phase was separated, and spin-dried to obtain a crude product, which was then washed with ethyl acetate / petroleum ether (1 : 4) Carry out recrystallization to obtain purer N-propargyl quinoxalinone.
[0074] Preparation of 1-propargyl-3-(1-m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com