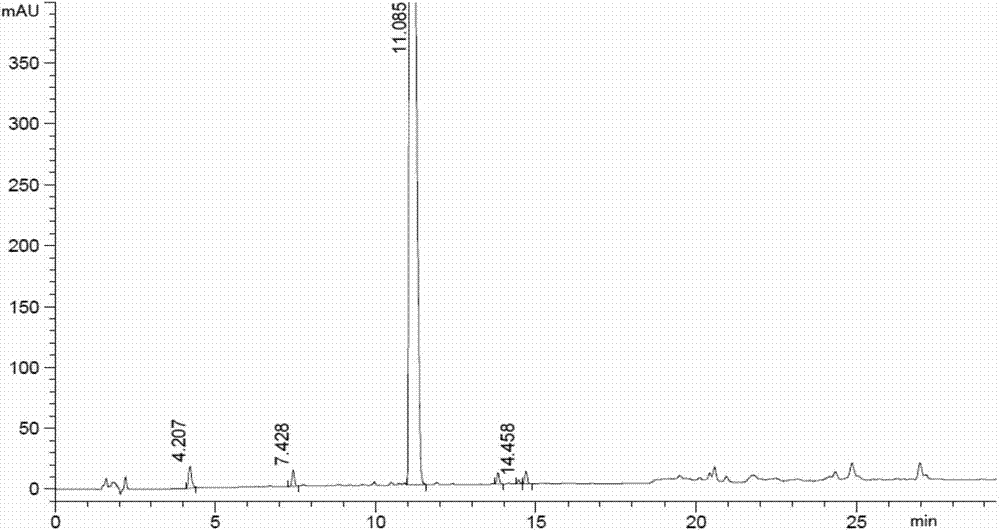
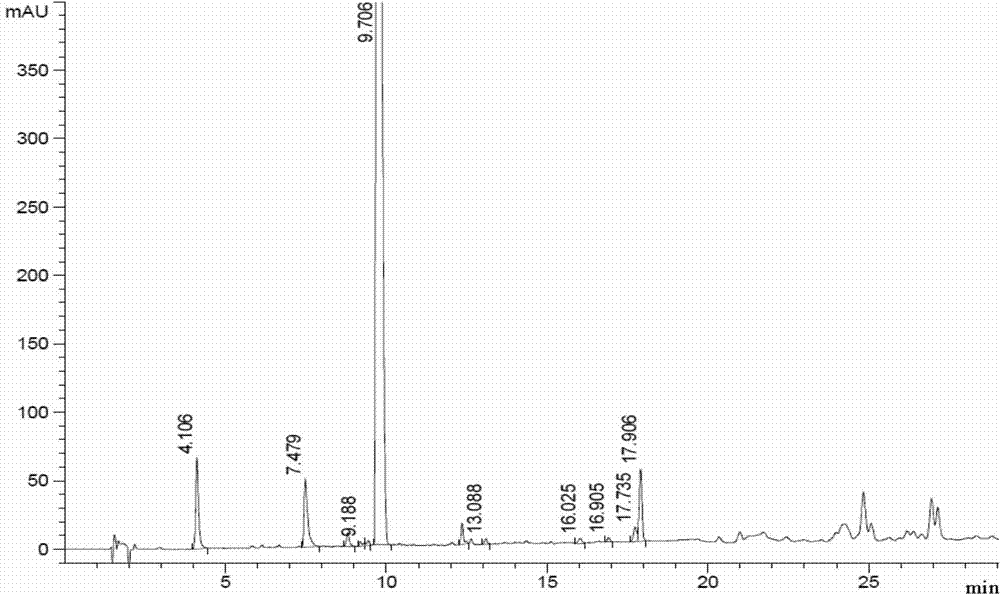
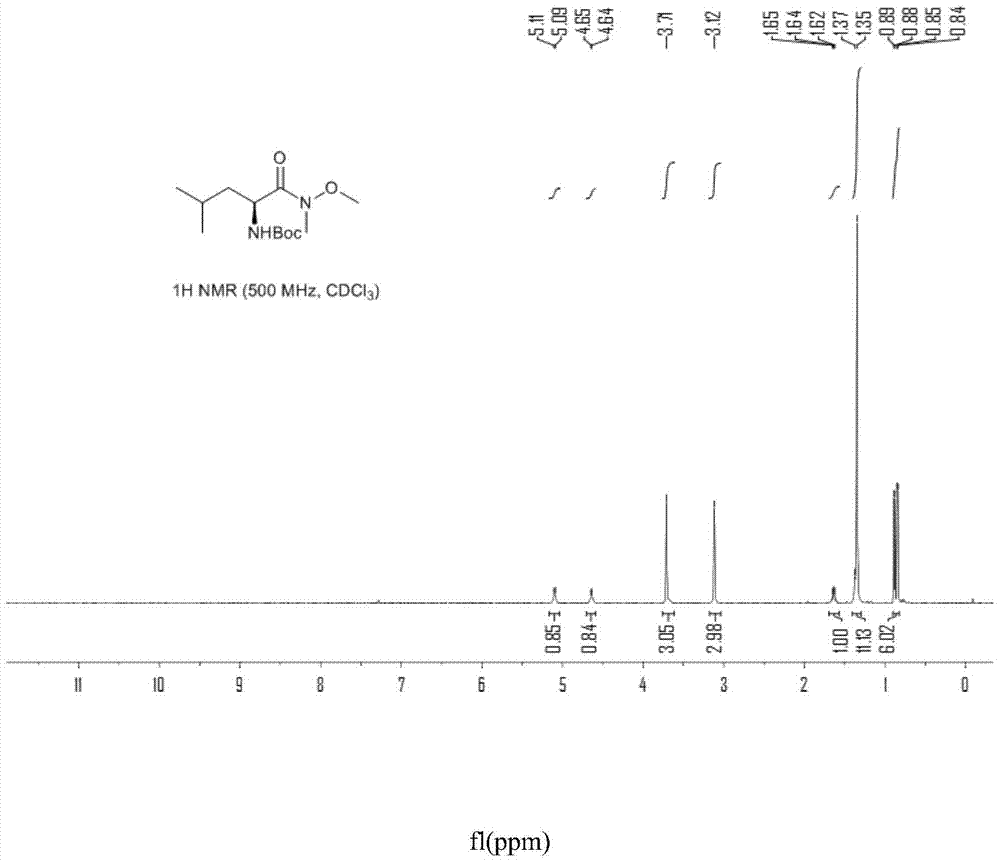
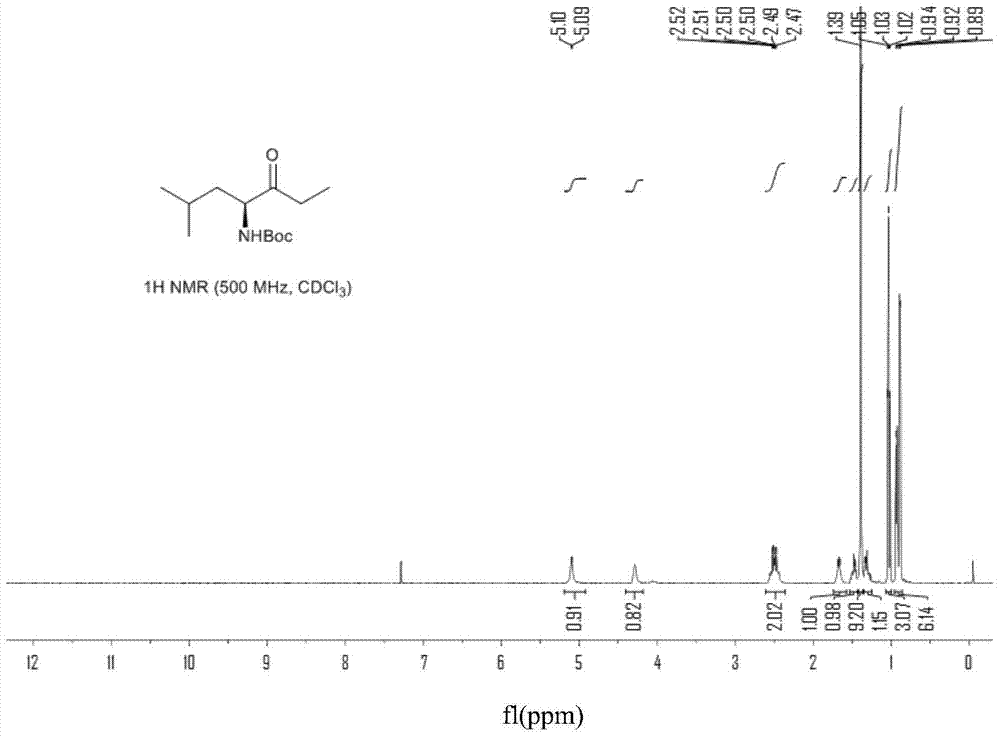
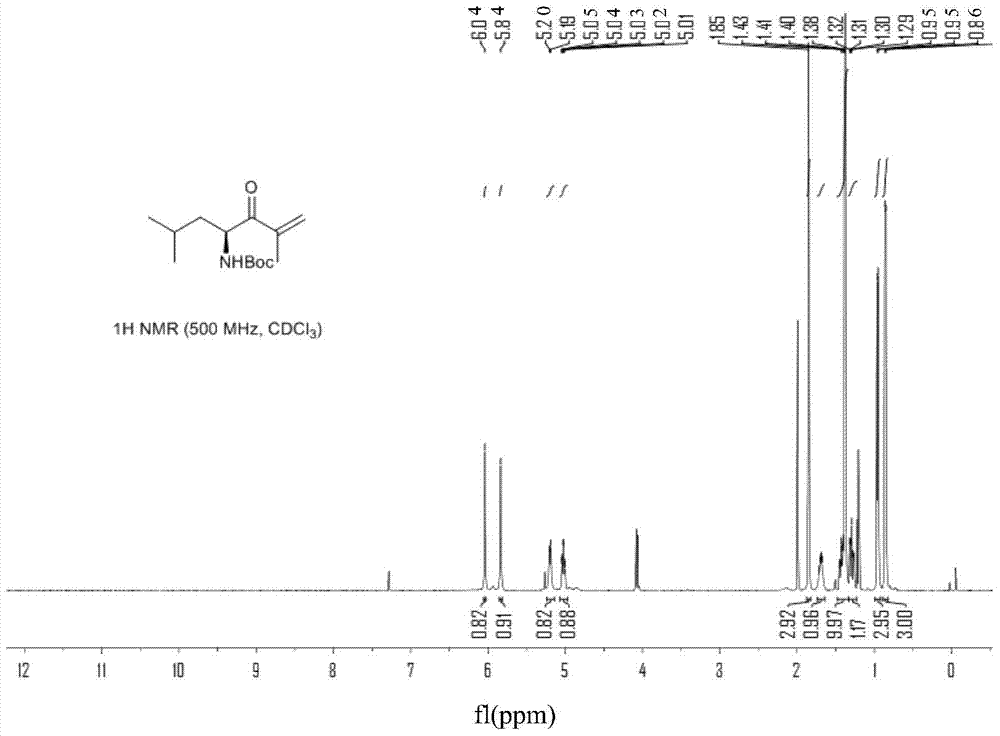
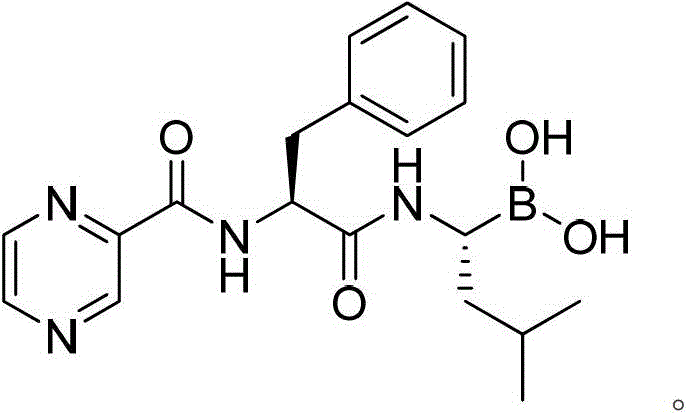
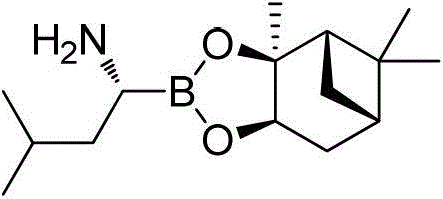
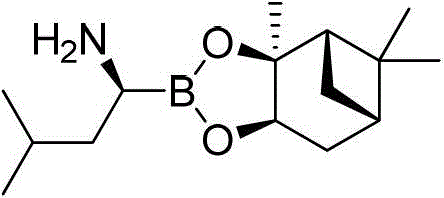
Patents
Literature
39 results about "Zolmitriptan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
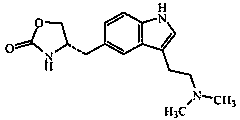
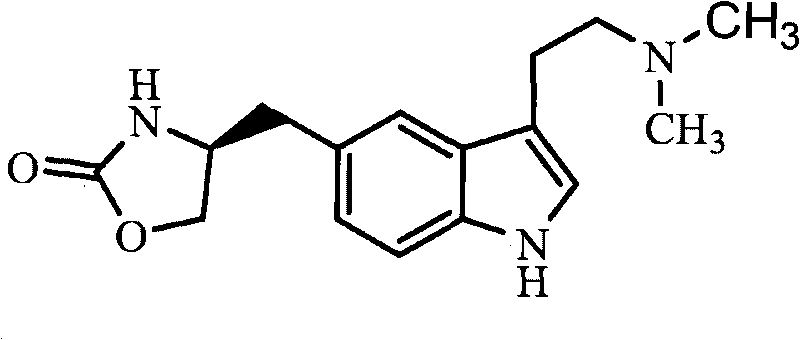
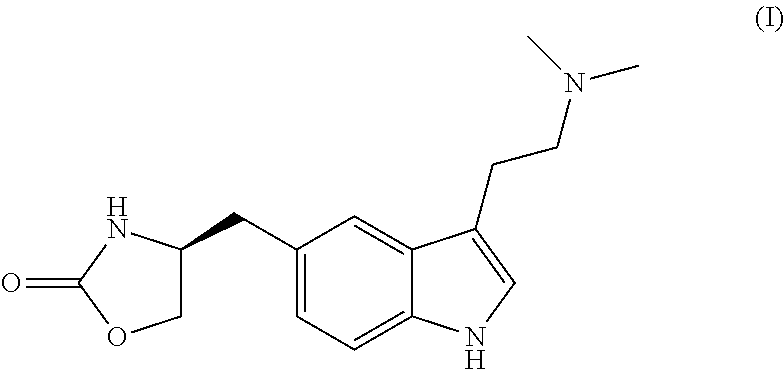
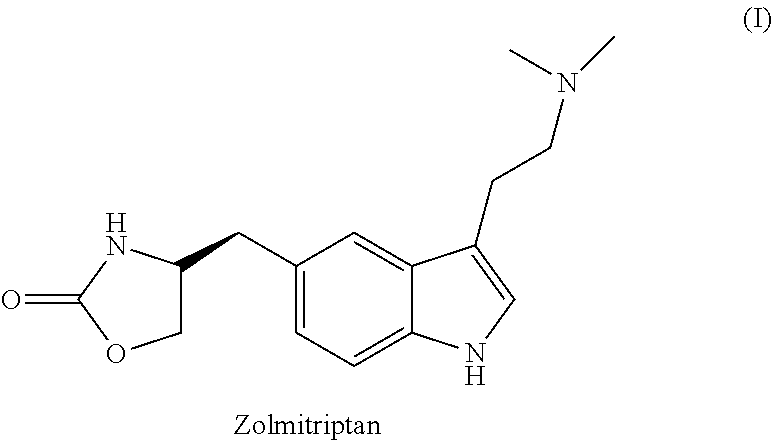
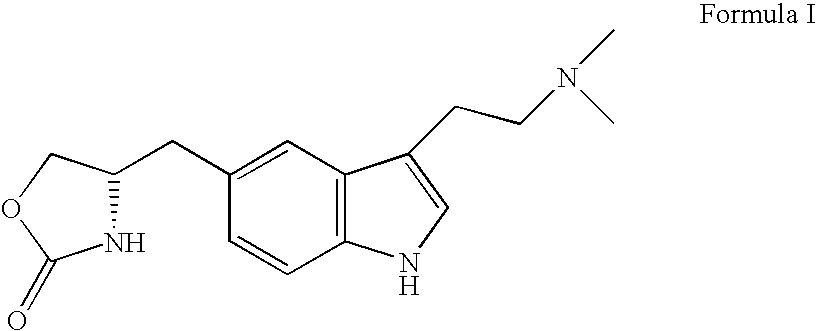
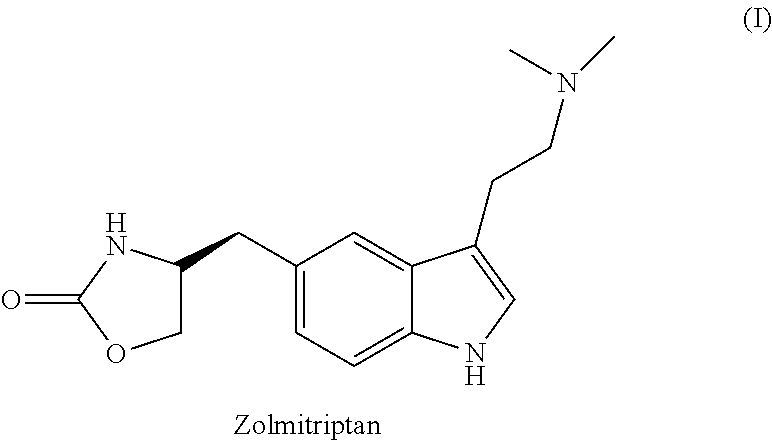
Zolmitriptan is used to treat migraines.
Use of BIBN4096 in combination with other antimigraine drugs for the treatment of migraine
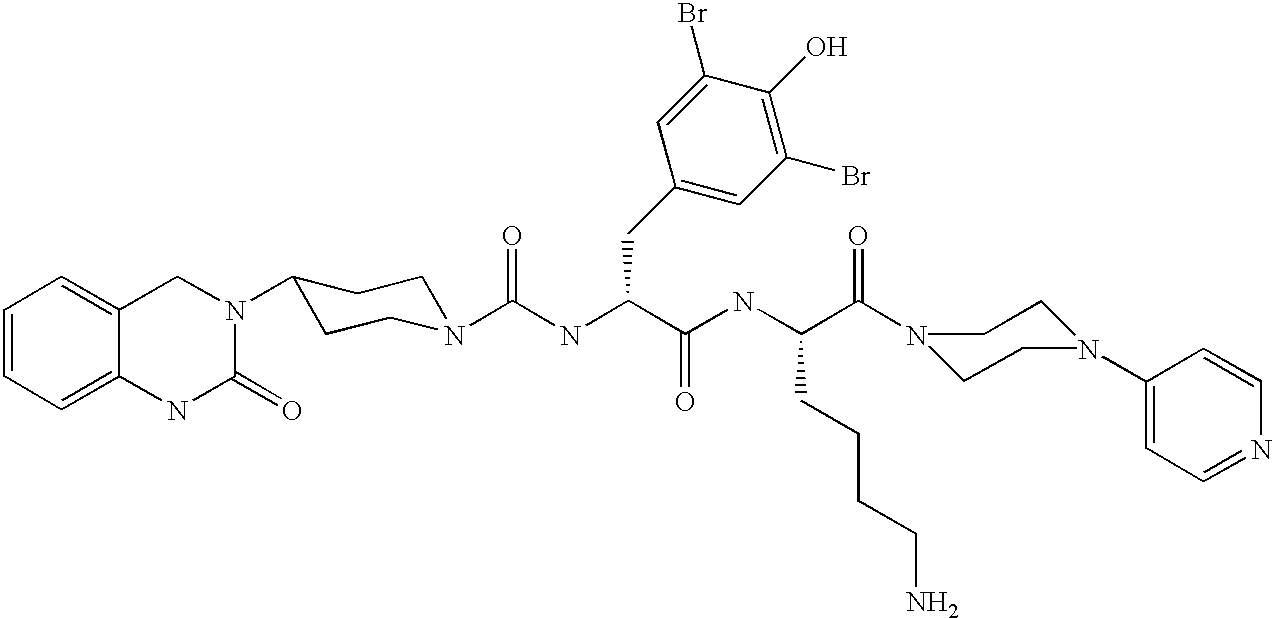
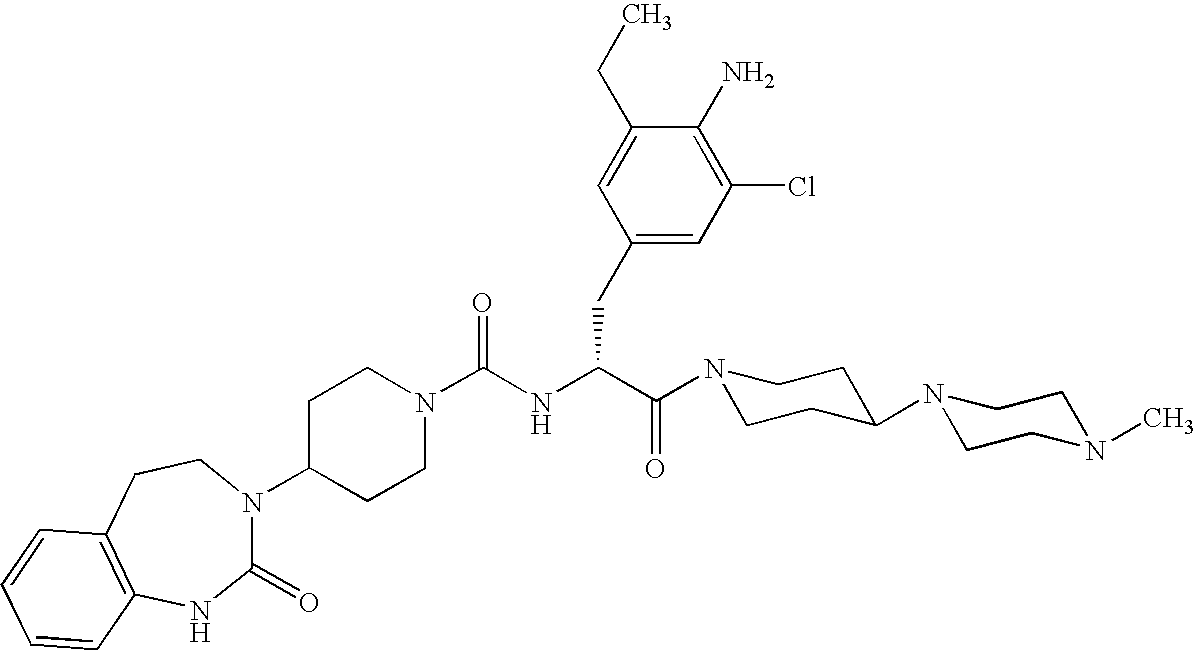
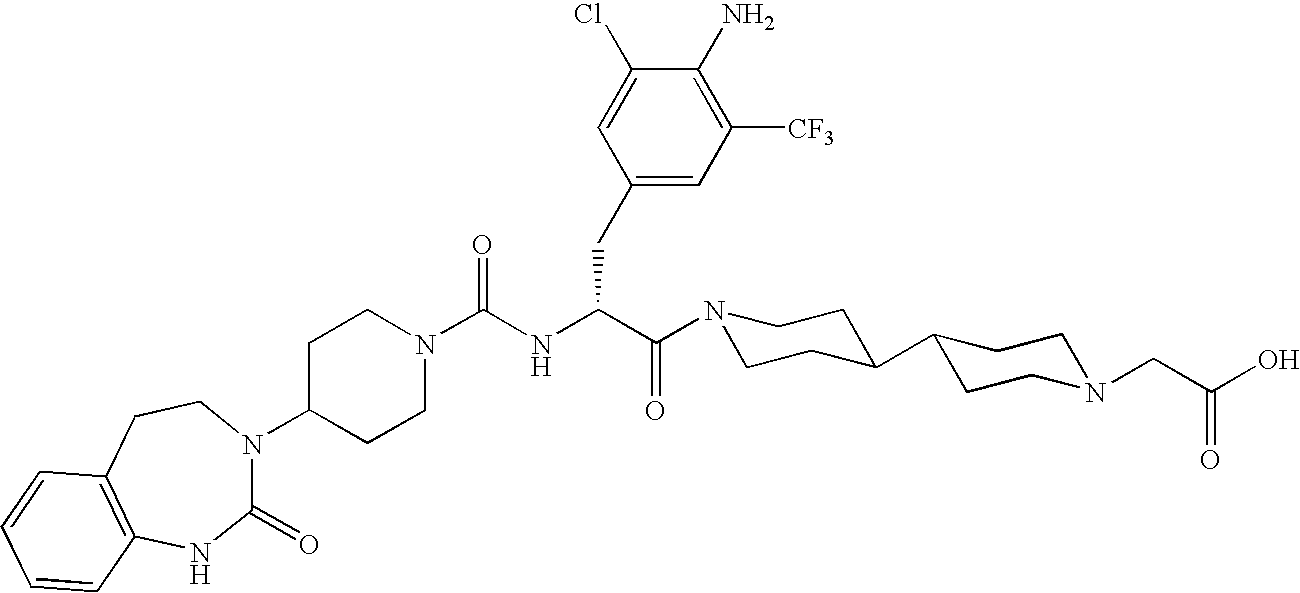
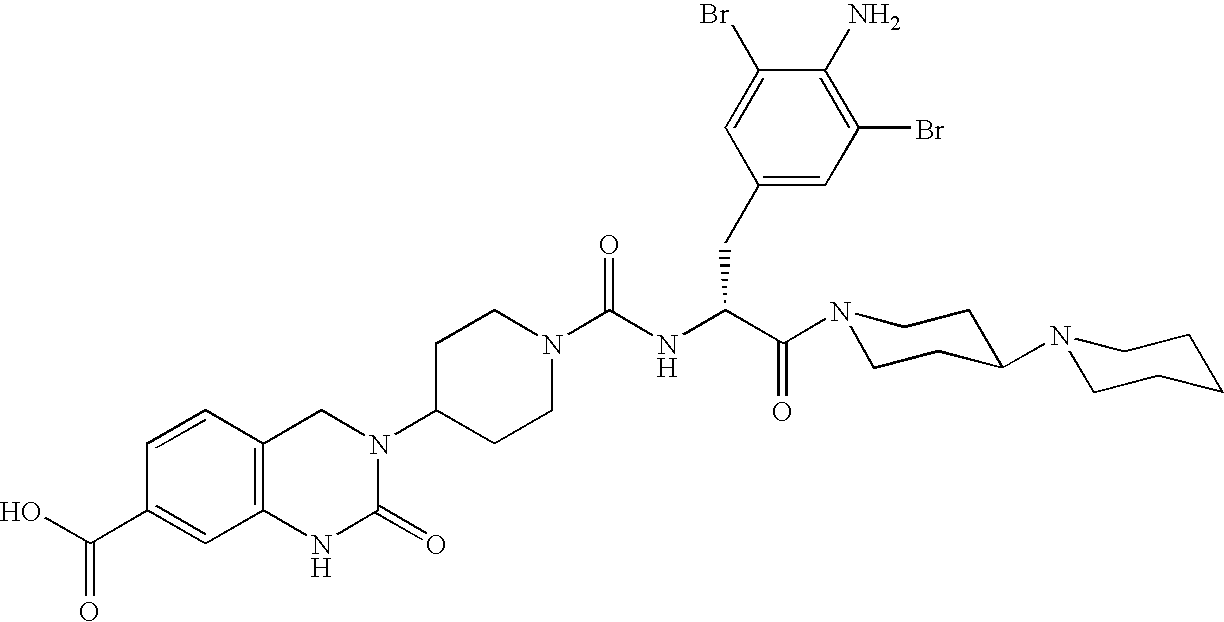
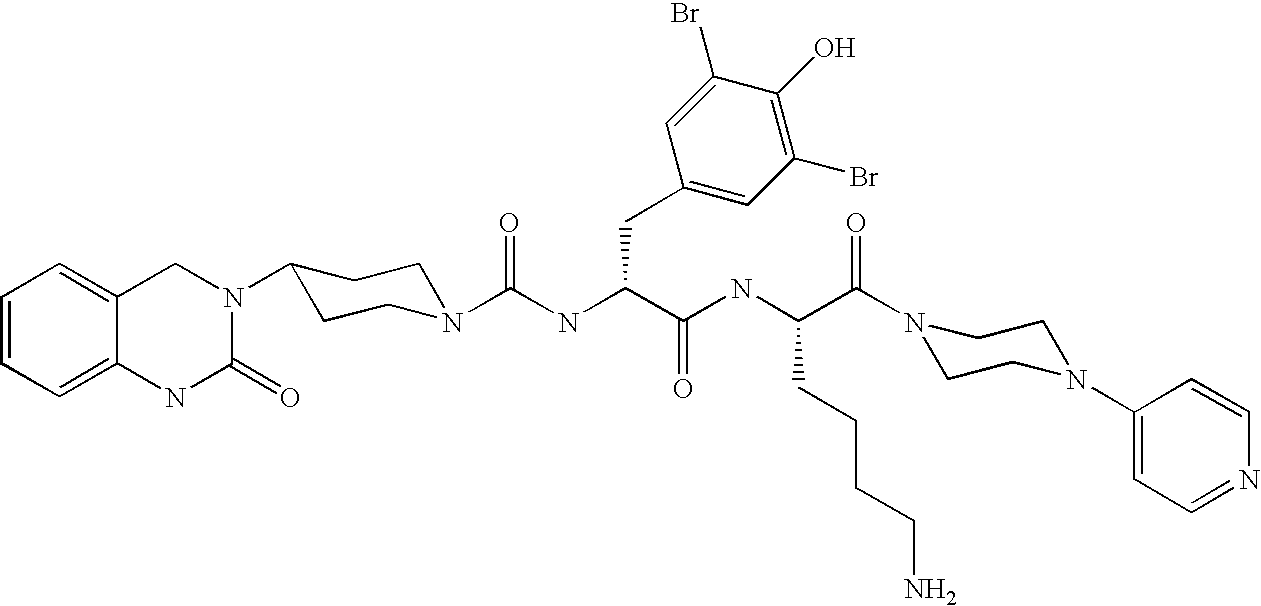
A method of treatment or prevention of headache, migraine or cluster headaches, which method comprises co-administration of a therapeutically effective amount of the compound 1-[N<2>-[3,5-dibromo-N-[[4-(3,4-dihydro-2(1H)-oxochinazolin-3-yl)-1-piperidinyl]-carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine[BIBN4096BS] or a physiologically acceptable salt thereof and a therapeutically effective amount of a second active antimigraine drug, particularly sumatriptan, zolmitriptan or dihydroergotamin or a physiologically acceptable salt thereof, as well as to the corresponding pharmaceutical compositions and the preparation thereof.
Owner:BOEHRINGER INGELHEIM PHARM KG
Use of selected CGRP-antagonists in combination with other antimigraine drugs for the treatment of migraine
The present invention relates to a process for the treatment or prevention of indications which are selected from among the group comprising headaches, migraine and cluster headaches, this process comprising the joint administration of a therapeutically effective amount of a selected CGRP antagonist (A), a physiologically acceptable salt thereof or a hydrate of the salt and a therapeutically effective amount of a second or third active anti-migraine medicament (B), particularly sumatriptan, zolmitriptan or dihydroergotamine or a physiologically acceptable salt thereof, and to the corresponding pharmaceutical compositions and the preparation thereof.
Owner:BOEHRINGER INGELHEIM INT GMBH
Use of selected CGRP-antagonists in combination with other antimigraine drugs for the treatment of migraine
The present invention relates to a process for the treatment or prevention of indications which are selected from among the group comprising headaches, migraine and cluster headaches, this process comprising the joint administration of a therapeutically effective amount of a selected CGRP antagonist (A), a physiologically acceptable salt thereof or a hydrate of the salt and a therapeutically effective amount of a second or third active anti-migraine medicament (B), particularly sumatriptan, zolmitriptan or dihydroergotamine or a physiologically acceptable salt thereof, and to the corresponding pharmaceutical compositions and the preparation thereof.
Owner:RUDOLF KLAUS +8
Use of BIBN4096 in combination with other antimigraine drugs for the treatment of migraine
InactiveUS20060183693A1Nervous disorderPeptide/protein ingredientsCo administrationAntimigraine drug
A method of treatment or prevention of headache, migraine or cluster headaches, which method comprises co-administration of a therapeutically effective amount of the compound 1-[N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2(1H)-oxochinazolin-3-yl)-1-piperidinyl]-carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine [BIBN4096BS] or a physiologically acceptable salt thereof and a therapeutically effective amount of a second active antimigraine drug, particularly sumatriptan, zolmitriptan or dihydroergotamin or a physiologically acceptable salt thereof, as well as to the corresponding pharmaceutical compositions and the preparation thereof.
Owner:BOEHRINGER INGELHEIM PHARMA KG
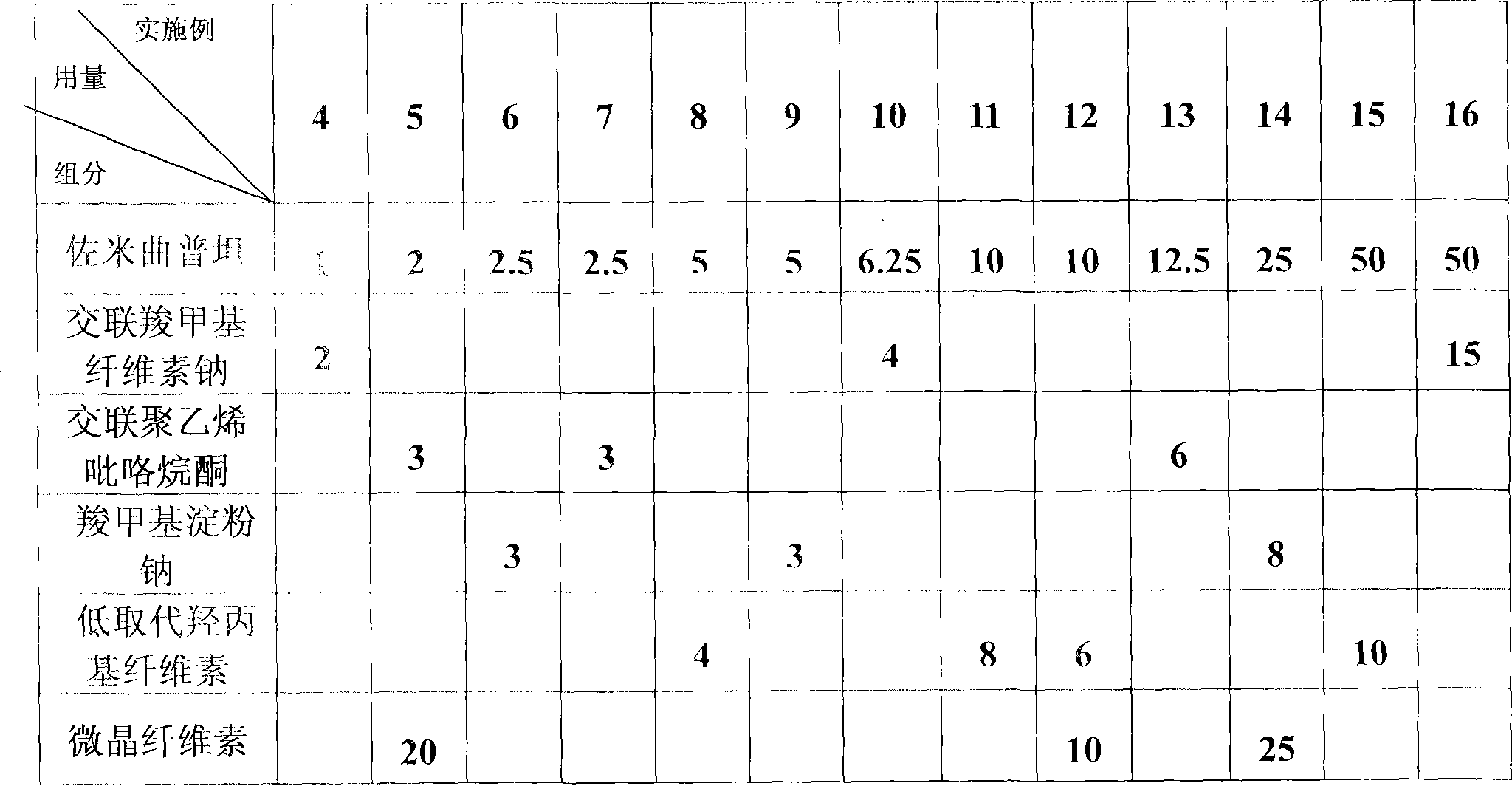
Zolmitriptan quick-release formulation
ActiveCN1634043ADisintegrates quicklyRapid dissolutionOrganic active ingredientsNervous disorderAdjuvantMedicine
The invention provides a Zolmitriptan quick-release formulation which can further improve the dissolving degree of the main medicament, greatly increase the absorbing velocity of the medicament in stomach and intestine, thus making the curative effect exert completely. Based on the physics and chemistry nature of the Zolmitriptan, adjuvant having specific disintegration and dissolving boosting actions for grease solving Zolmitriptan is selected from a plurality of medicinal findings through experiment.
Owner:LUNAN PHARMA GROUP CORPORATION
Zolmitriptan tongue tablet
InactiveCN101181267AAvoid first pass effectAvoid enzymatic digestionOrganic active ingredientsNervous disorderTreatment choicesSuperior vena caval
The invention provides a zolmitriptan sublingual tablet, which is characterized in that it contains the main drug zolmitriptan, a disintegrating agent and a filler. In every 1000 sublingual tablets, the content of zolmitriptan is 1 -50g, the content of disintegrating agent is 2-20g, the content of filler is 40-200g; it also contains 0-20g of corrective agent, 10-60g of binder and 0.5-5g of lubricant. The present invention utilizes the characteristics of non-keratinized sublingual mucosa, rich capillaries, and fast blood flow, aiming at the absorption of zolmitriptan ordinary tablets through the gastrointestinal tract, slow onset of action, first-pass effect, and low bioavailability. The deficiencies of advanced prior art, zolmitriptan is made into sublingual tablet, can make it absorb through sublingual mucosa, directly enter blood circulation through jugular vein and superior vena cava, take effect rapidly; Avoid oral administration The first-pass effect improves bioavailability and ensures curative effect. Moreover, water is not needed when taking the medicine, and it is placed under the tongue to contain it, so it is convenient to take and has a good taste. With the characteristics of rapid onset of action, convenient administration and high bioavailability, it can provide patients with a new treatment option and fill the gap in the market.
Owner:重庆医科大学医药研究所
Process for preparing optically pure zolmitriptan
A process for preparing zolmitriptan, proceeding through the intermediate Ethyl-3-[2-(1,3-dioxo-2,3-dihydro-1H-2-isoindoleyl)ethyl]-5-[(4S)-2-oxo-1,3-oxazolan-4-ylmethyl]-1H-2-indole carboxylate.
Owner:DR REDDYS LAB LTD
Chiral chromatographic fixed phase stuffing of vancomycin phenylisocyanate and its prepn
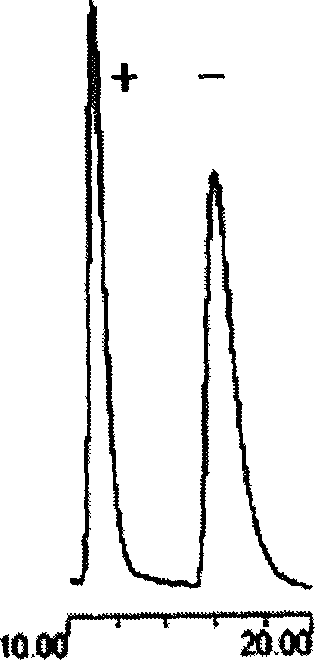
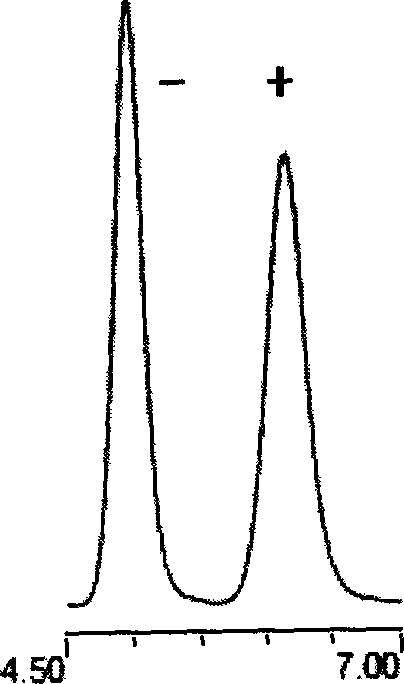
The chiral chromatographic fixed phase stuffing of vancomycin phenylisocyanate is prepared through bonding vancomycin as glycopeptide macrocyclic antiseptic chemical onto silicon gel carrier, and the subsequent derivation of phenylisocyanate to obtain chiral chromatographic fixed phase stuffing. The silicon gel carrier is first reacted with 3-aminopropyl triethoxy silane for silanation and then space arm activated; and the activated silicon gel carrier is bonded with vancomycin and finally derivated with phenylisocyanate. The chiral chromatographic fixed phase stuffing of vancomycin phenylisocyanate after assembled to column may be used in forward phase, reverse phase and polar organic phase chromatographic condition. The present invention may be used in simple polar organic phase mode to resolve the medicine antimer of zolmitriptan, lamivudine, etc.
Owner:ZHEJIANG UNIV
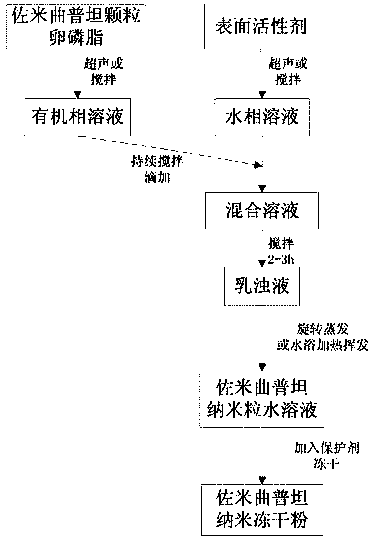
Lyophilized zolmitriptan nanometer powder and preparation method thereof
InactiveCN104800191AGood biocompatibilityLow toxicity in vivoOrganic active ingredientsNervous disorderDispersityLipid formation
The invention discloses lyophilized zolmitriptan nanometer powder and a preparation method thereof. The lyophilized powder consists of the following raw materials in parts by weight: 1 part of zolmitriptan, 6-14 parts of carrier materials, and 90-130 parts of a surfactant, wherein the carrier materials adopt one or more of the following materials: lecithin, stearic acid, monoglyceride, chitosan and poloxamer. The carrier materials and the surfactant, which are selected by the lyophilized zolmitriptan nanometer powder disclosed by the invention are ideal auxiliary materials, because the biocompatibility is good, and the internal toxicity is low. Through the adoption of the method disclosed by the invention, the zolmitriptan is wrapped in solid lipid, after the wrapped zolmitriptan is lyophilized, the degree of dispersion is increased, the stability is improved, the bioavailability of zolmitriptan is improved, and the potency can be used in the level of nanometer. Organic solvents are removed, so that the residue of organic solvents can be avoided; the lyophilized zolmitriptan nanometer powder disclosed by the invention can be directly made into an oral agent of lyophilized powder, and can be further made into granules, tablets or capsules, which are used in clinical medicine.
Owner:徐俊 +1
Zolmitriptan quick-release formulation
ActiveCN100341504CDisintegrates quicklyRapid dissolutionOrganic active ingredientsNervous disorderAdjuvantMedicine
The invention provides a Zolmitriptan quick-release formulation which can further improve the dissolving degree of the main medicament, greatly increase the absorbing velocity of the medicament in stomach and intestine, thus making the curative effect exert completely. Based on the physics and chemistry nature of the Zolmitriptan, adjuvant having specific disintegration and dissolving boosting actions for grease solving Zolmitriptan is selected from a plurality of medicinal findings through experiment.
Owner:LUNAN PHARMA GROUP CORPORATION
Method of rapidly achieving therapeutic concentrations of triptans for treatment of migraines
ActiveUS9918932B2Fast concentrationOrganic active ingredientsPharmaceutical delivery mechanismHeadache severeSurgery
Compositions, devices and methods employing therapeutic concentrations of a triptan for treatment of migraine are described. Also described are methods and apparatuses for delivery of zolmitriptan for achieving a Tmax as quick as 2 minutes and not later than 30 minutes in the majority of subjects.
Owner:EMERGEX USA CORP
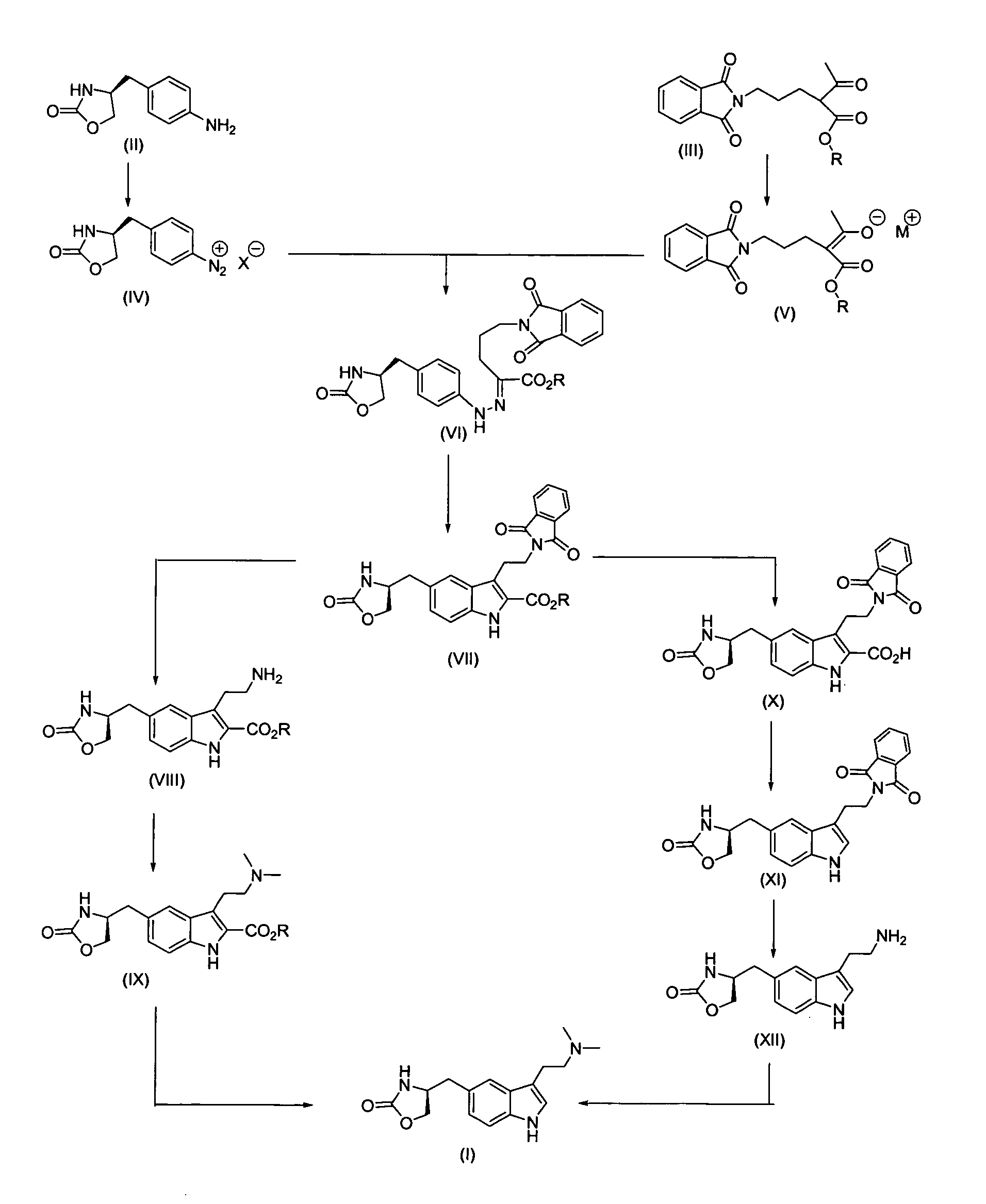
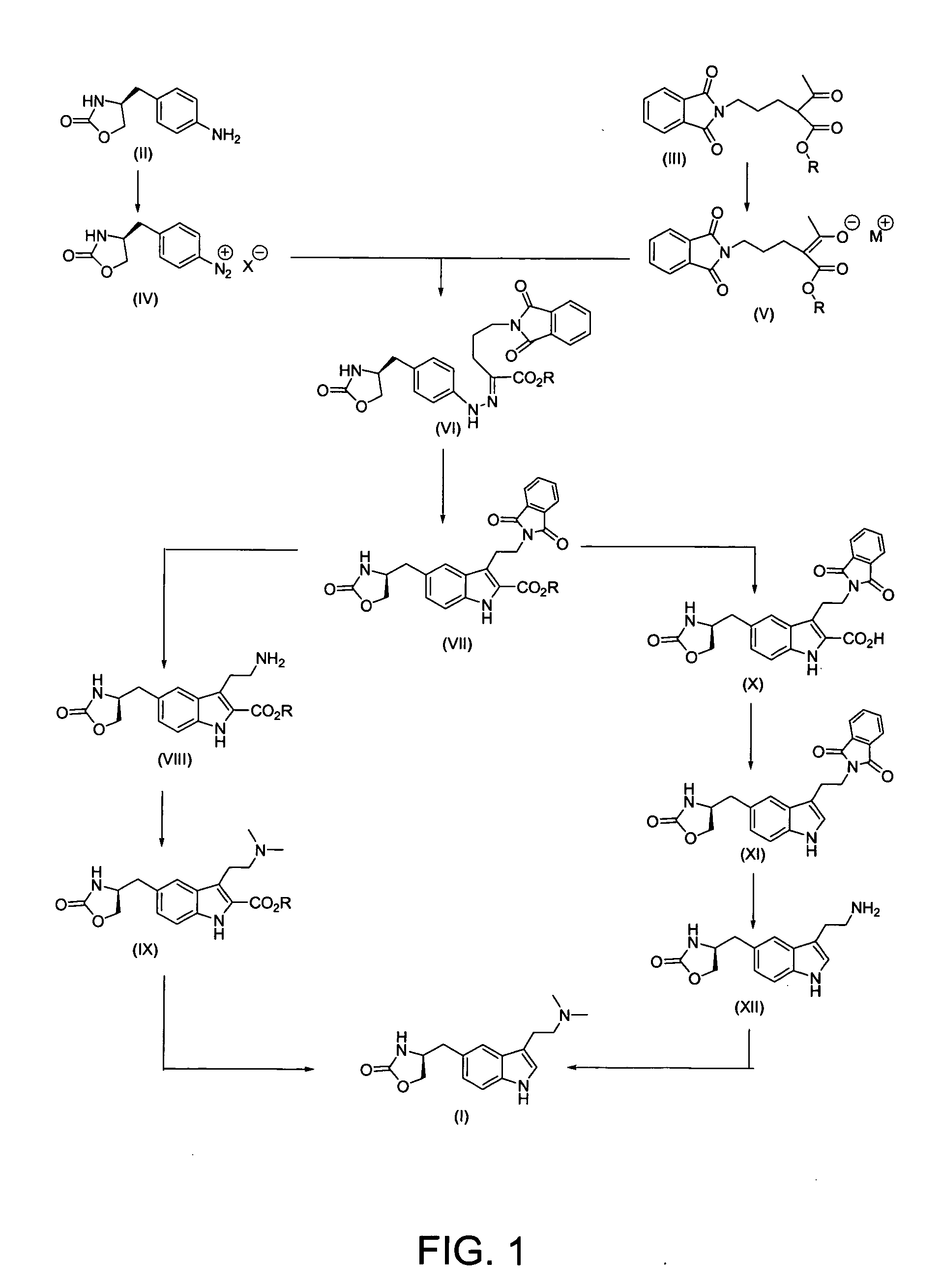
Process for preparing zolmitriptan compounds
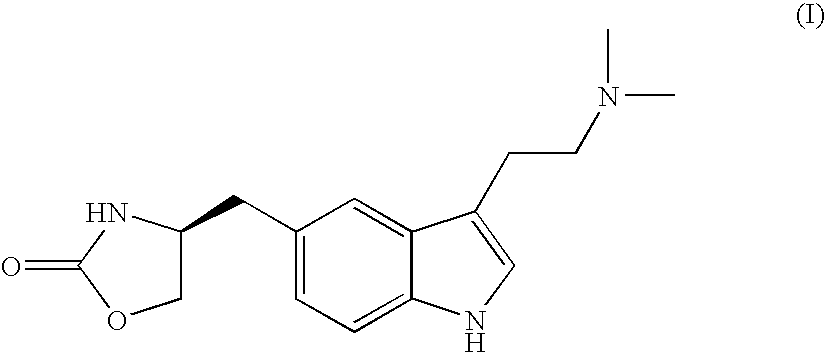
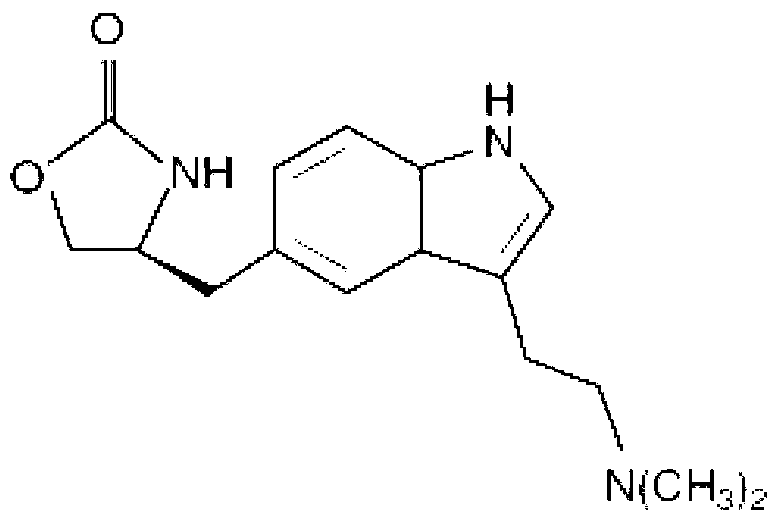
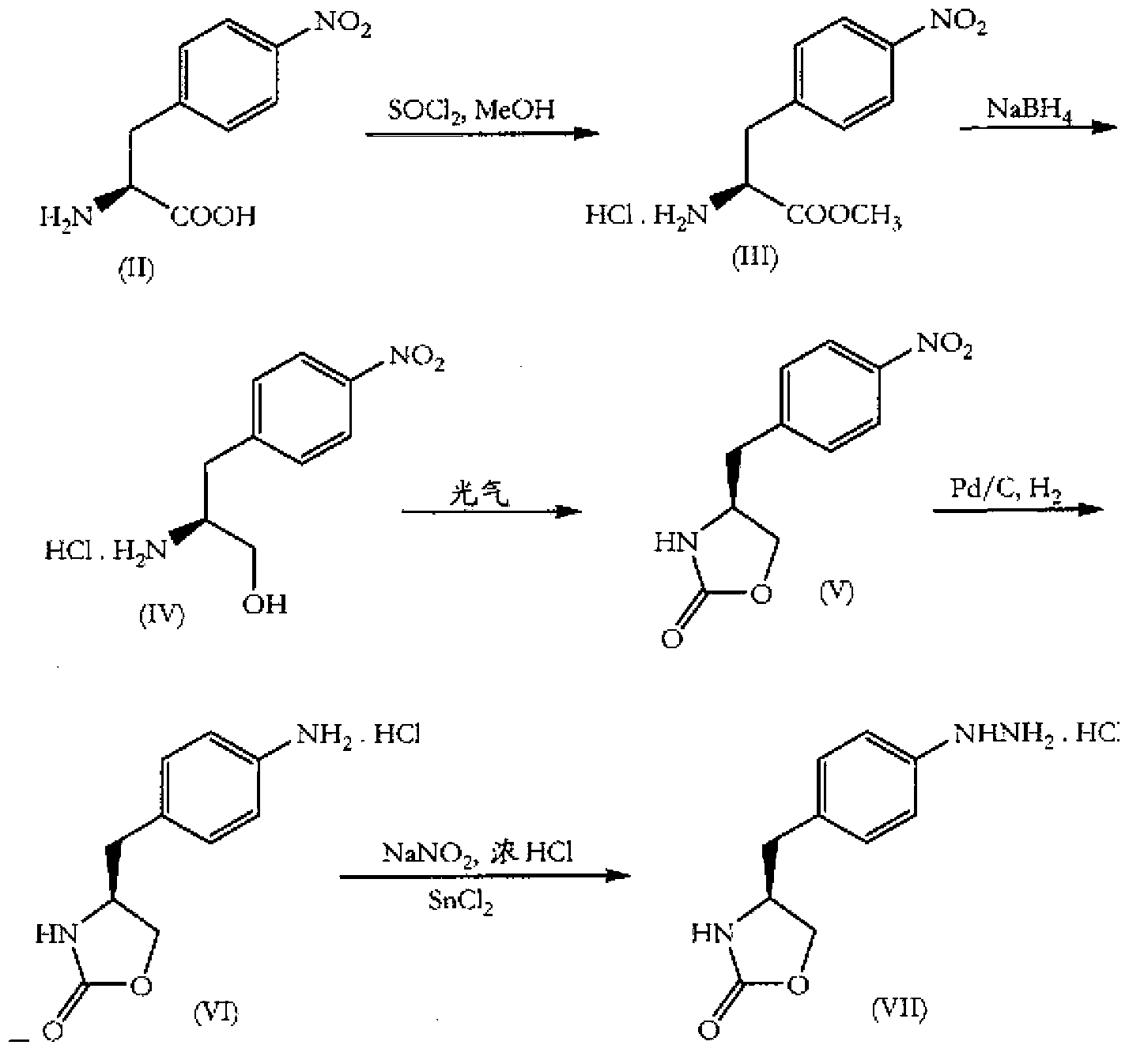
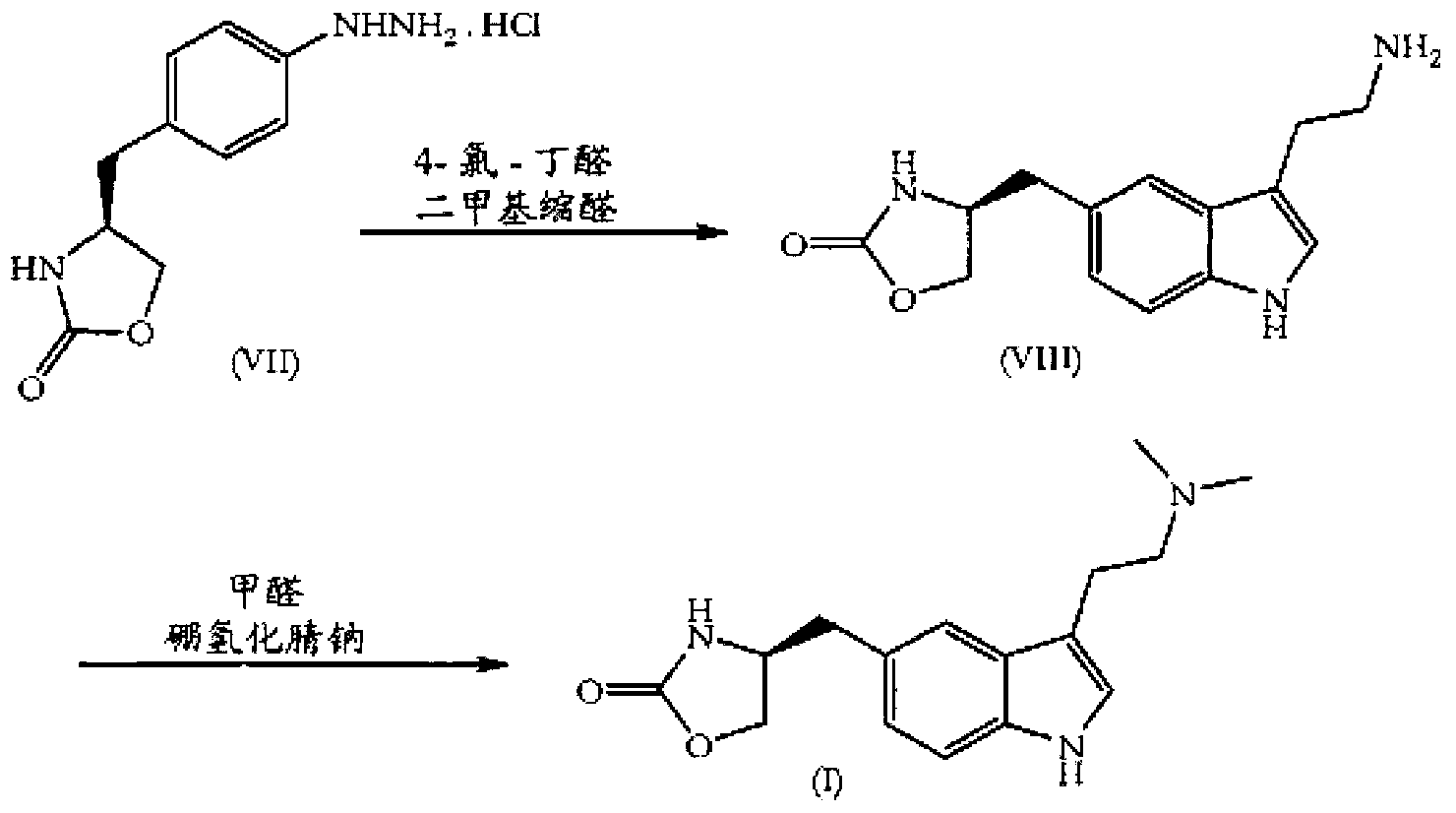
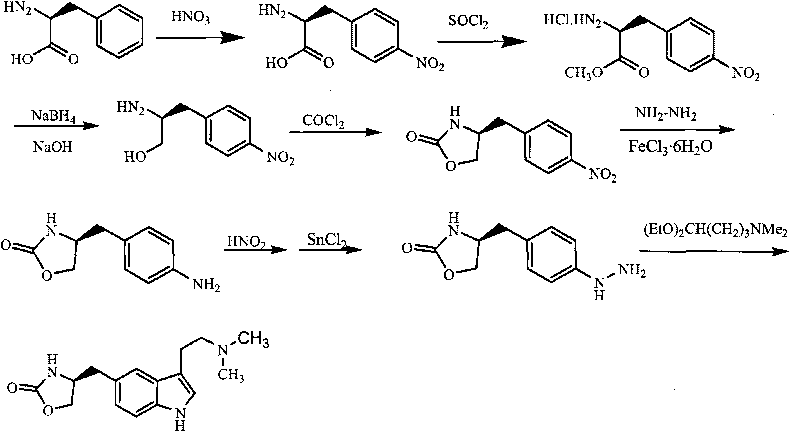
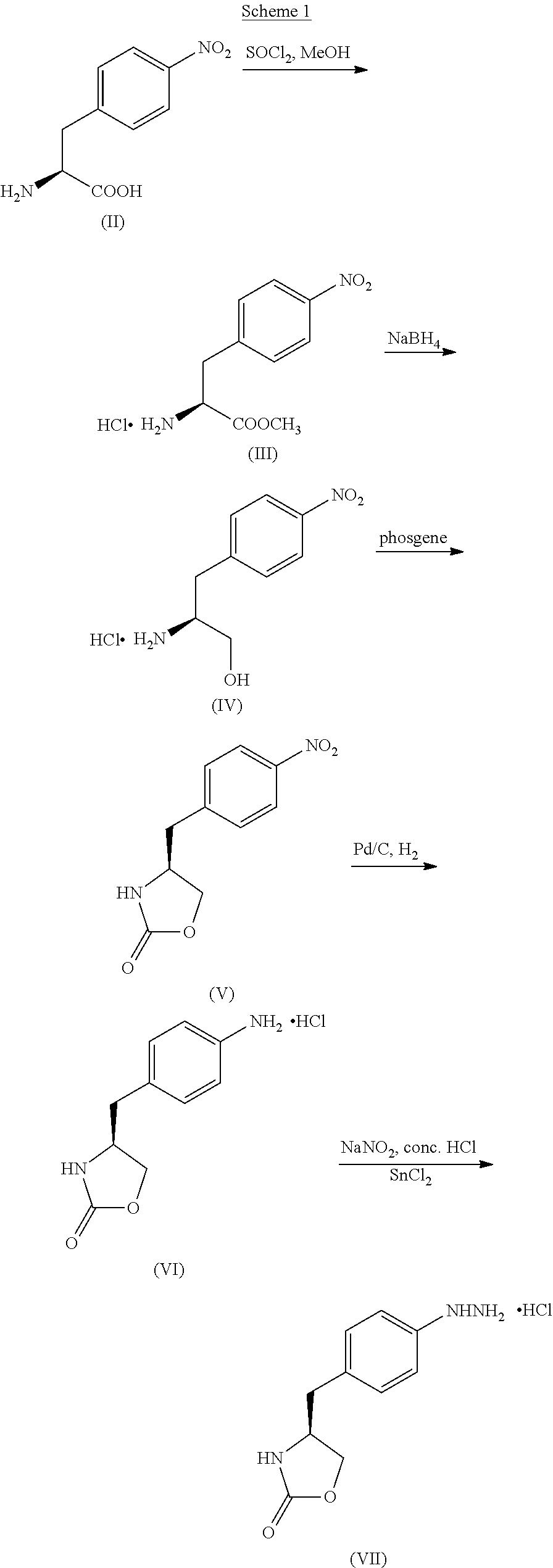
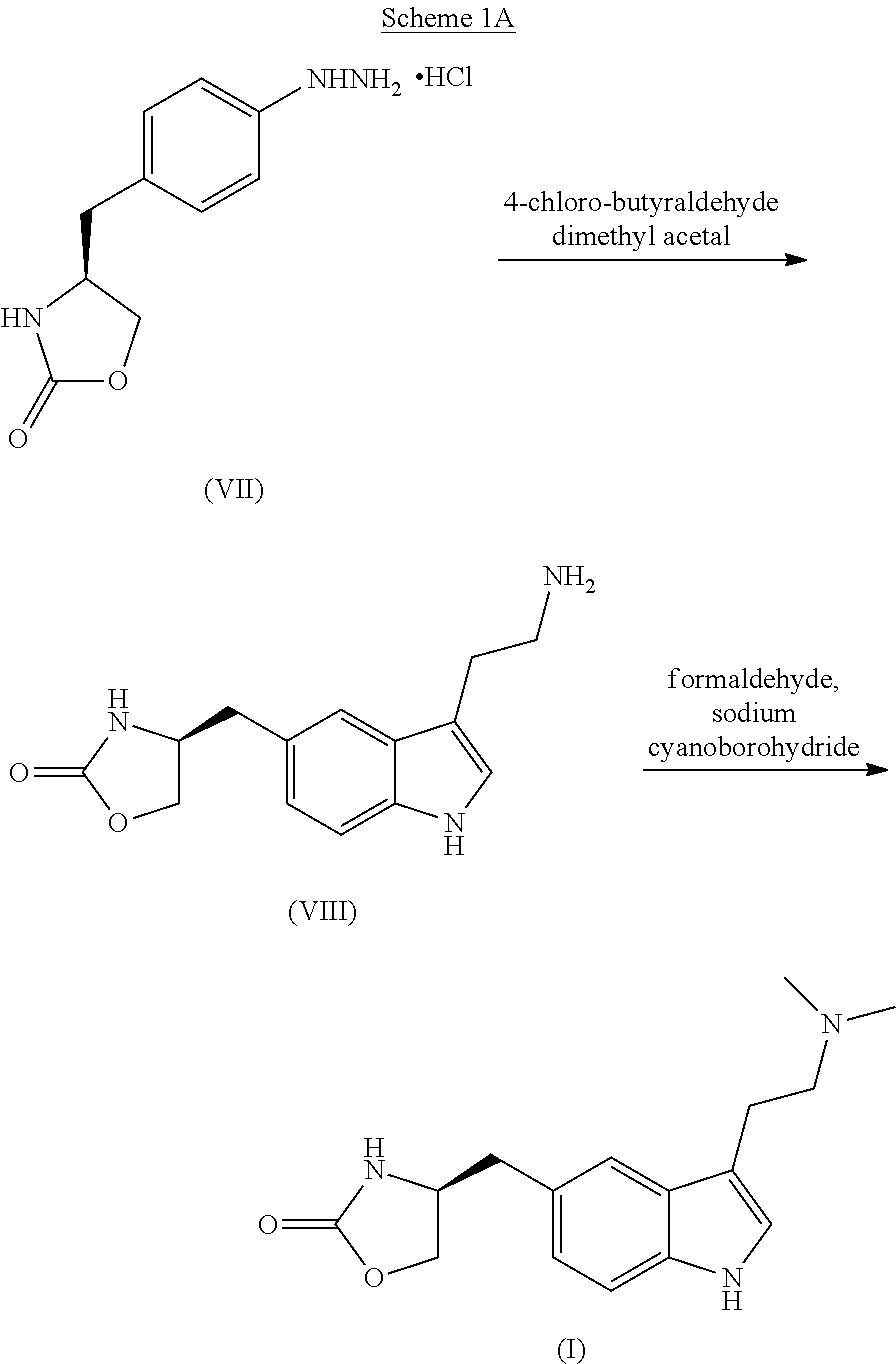
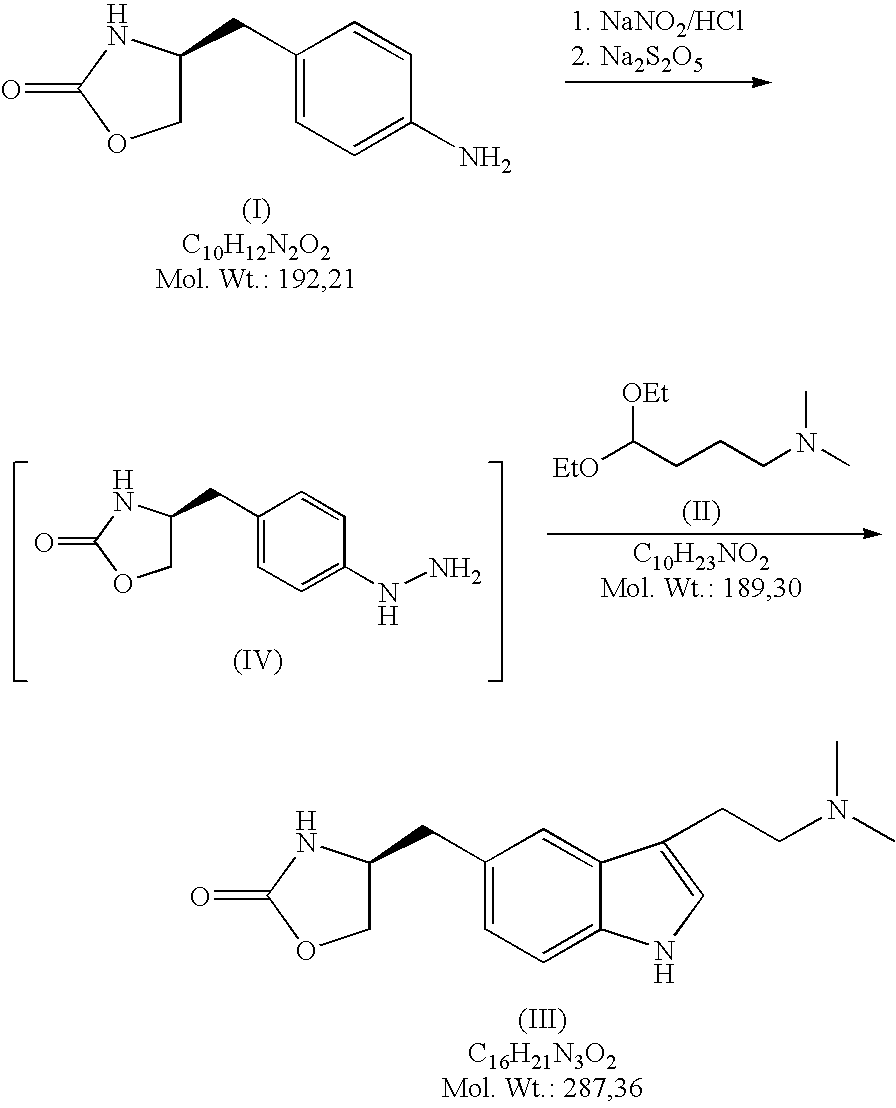
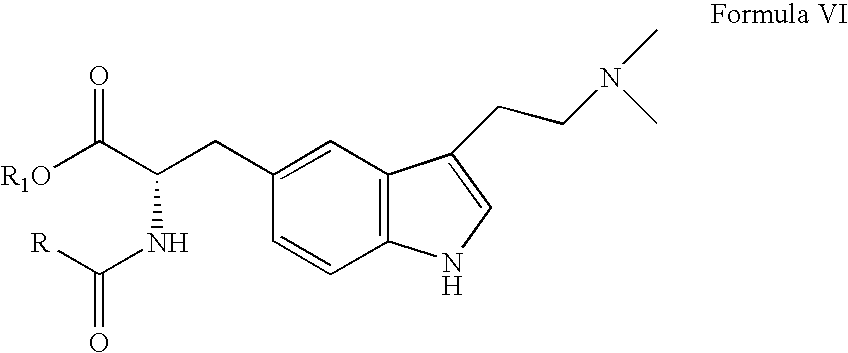
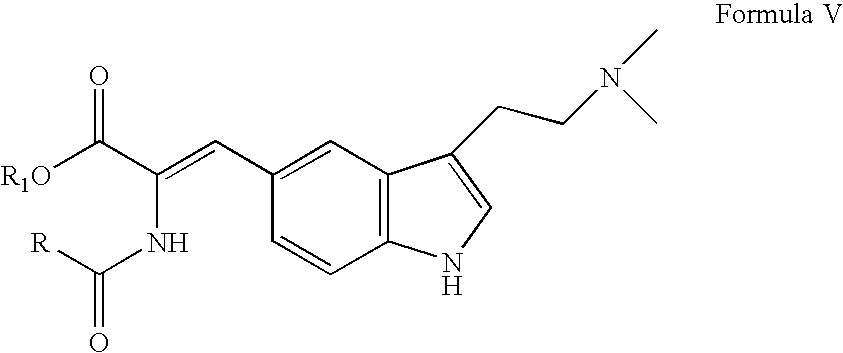
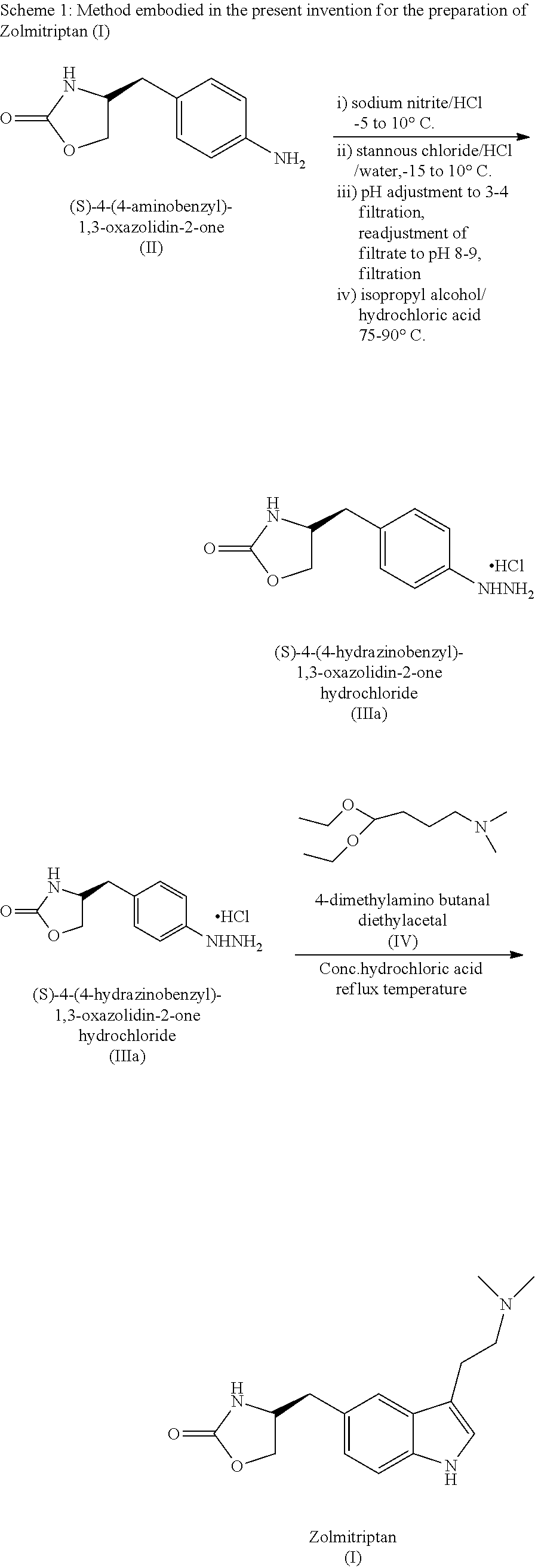
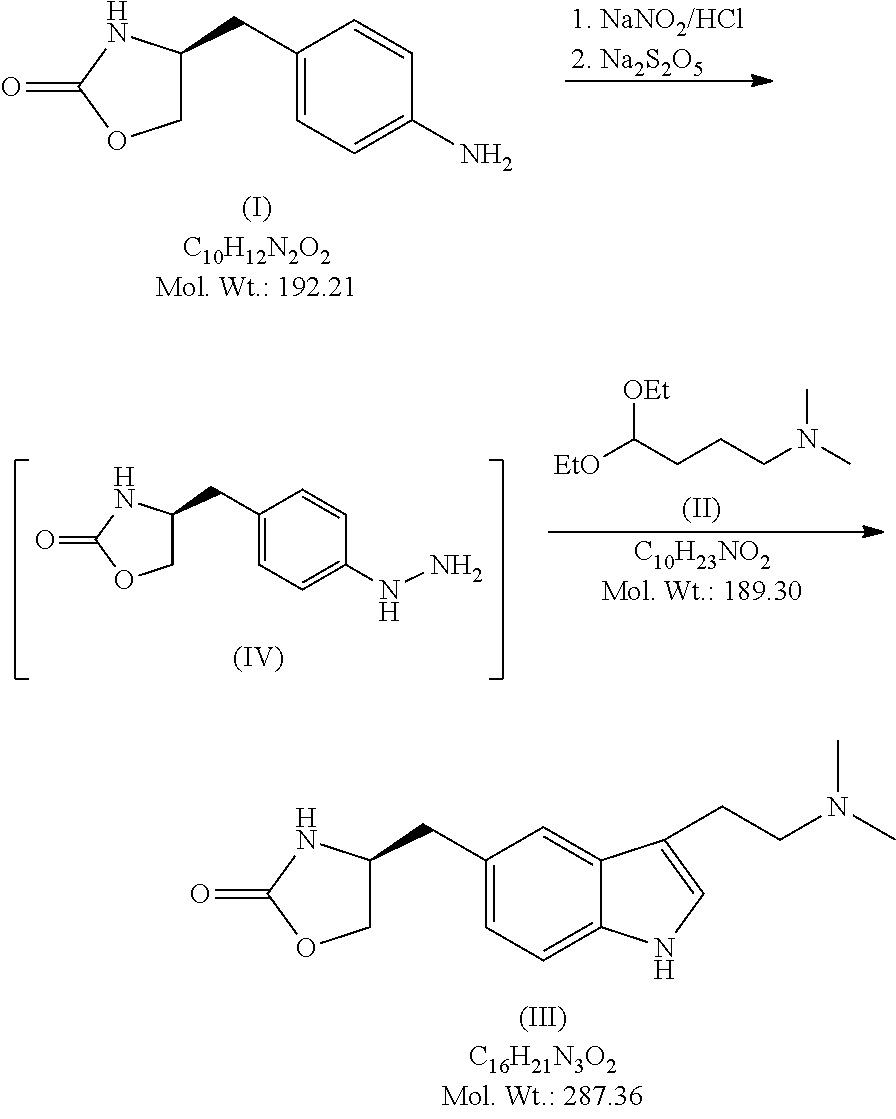
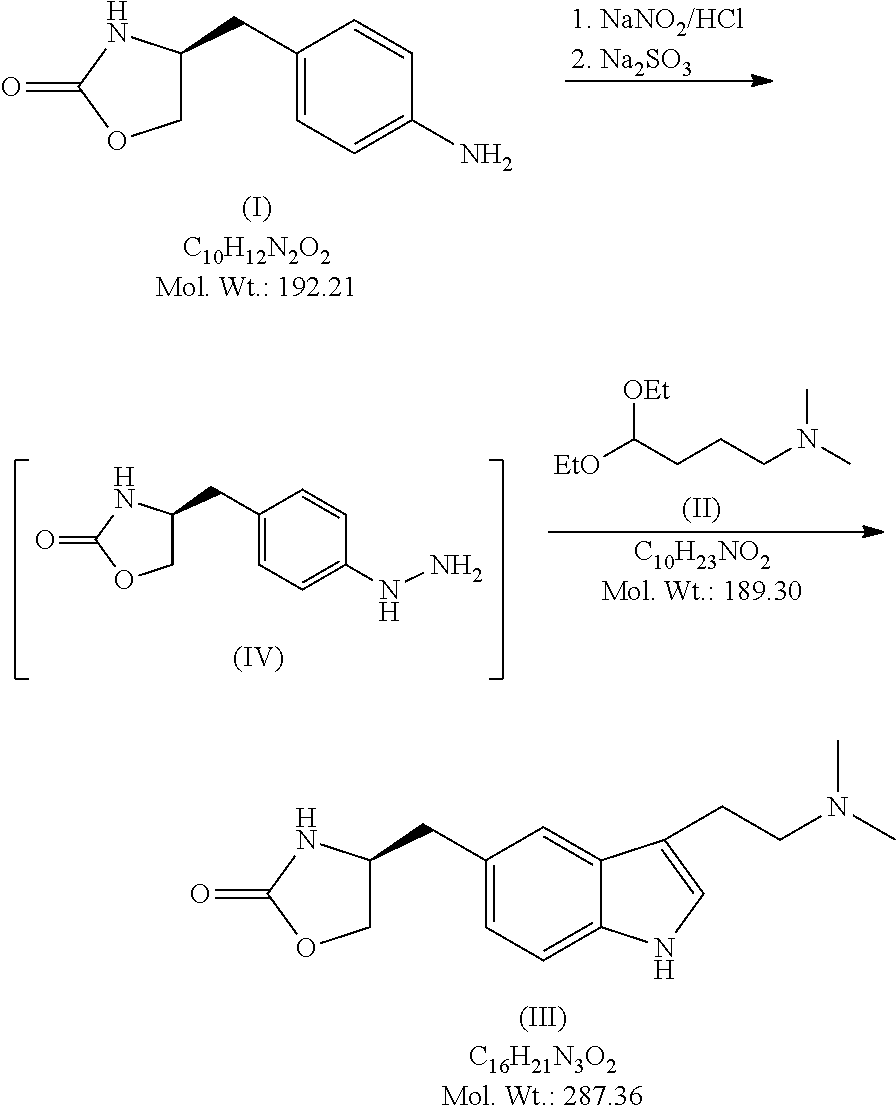
In particular, zolmitriptan or a pharmaceutically acceptable salt thereof, which includes a) Preparation of the diazonium salt of the aniline hydrochloride (II); followed by reduction and acidification to give hydrazine (III); b) In situ Reaction of the hydrazine hydrochloride (III) with α-keto-δ-valerolactone, to give the hydrazone (IV); c) Fischer indole synthesis of the hydrazone (IV), to give the pyranoindolone of formula (V); d) Transesterification of the pyranoindolone (V) to provide the compound (VI), in which R means a straight or branched C1-C4 alkyl; e) Conversion of the hydroxyl group of the compound (VI) into dimethylamino to give the indolecarboxylate (VII), in which R means a straight or branched C1-C4 alkyl; f) Saponification of the 2-carboalkoxy group of the compound (VII), to provide indolecarboxylic acid (VIII); g) Decarboxylation of the indolecarboxylic acid (VIII), to provide zolmitriptan and, eventually, to provide a pharmaceutically acceptable salt thereof.
Owner:INKE SA (ES)
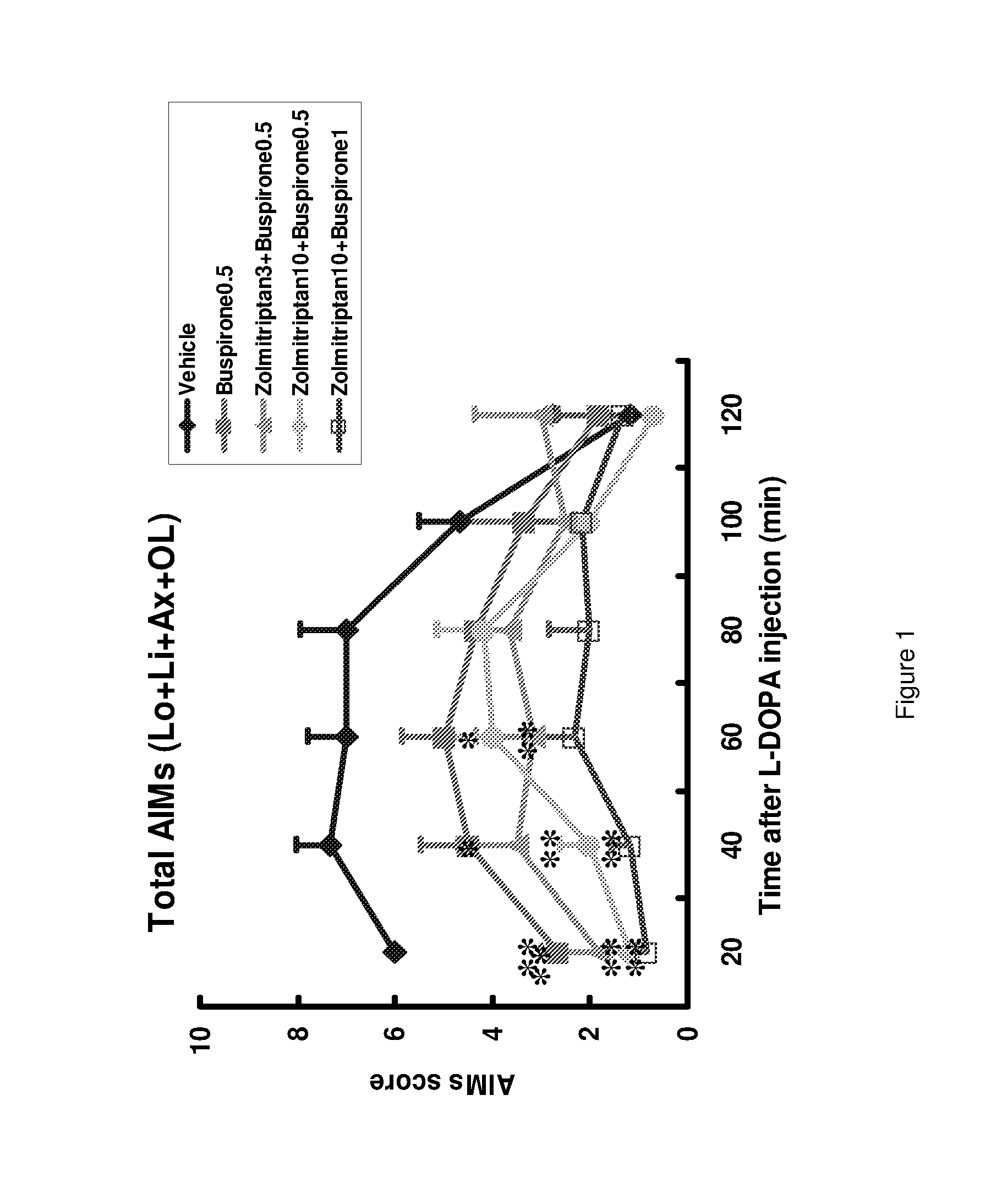
Orally available pharmaceutical formulation suitable for improved management of movement disorders
ActiveUS20150104506A1Good synergyGood curative effectOrganic active ingredientsNervous disorderImmediate releaseBULK ACTIVE INGREDIENT
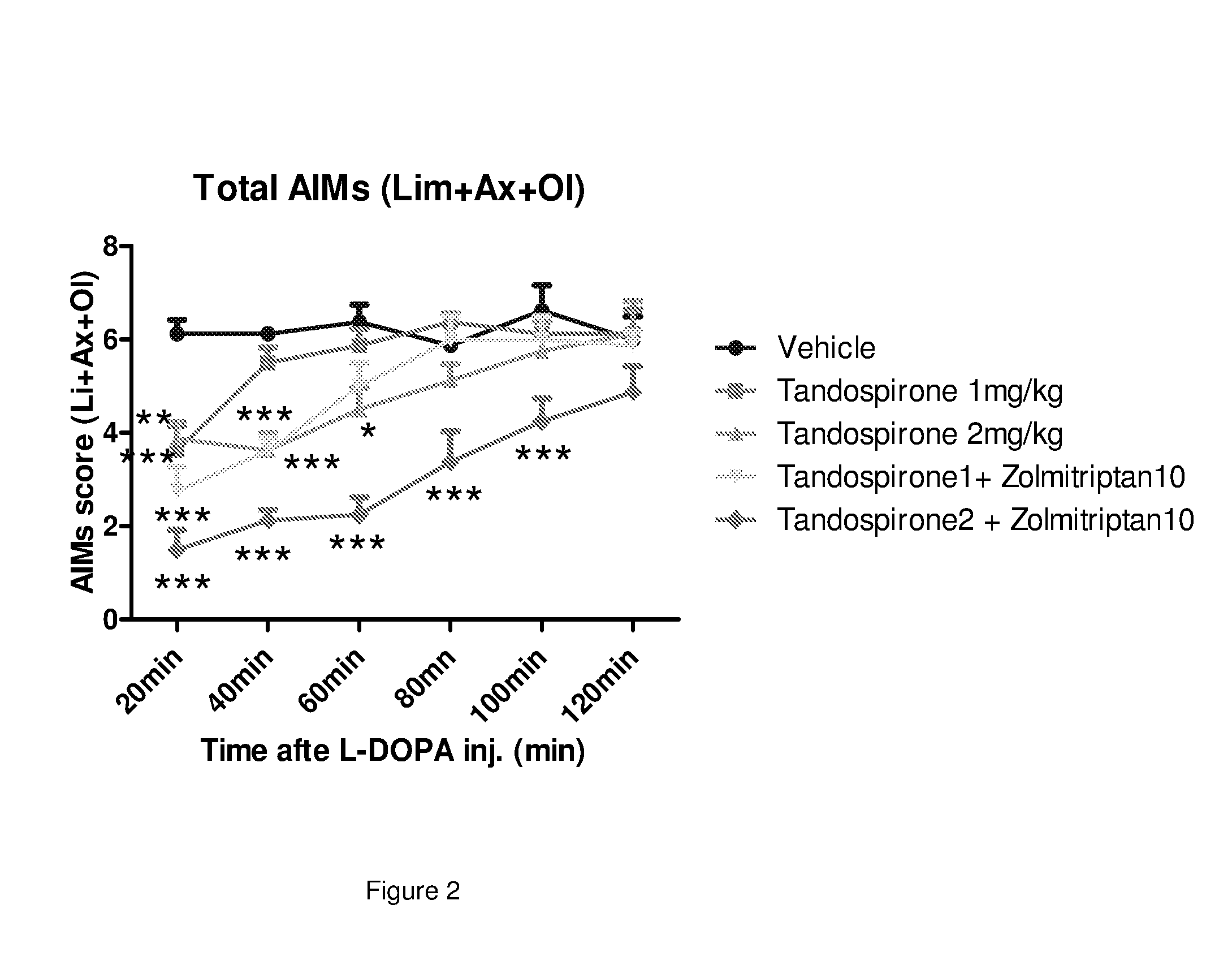
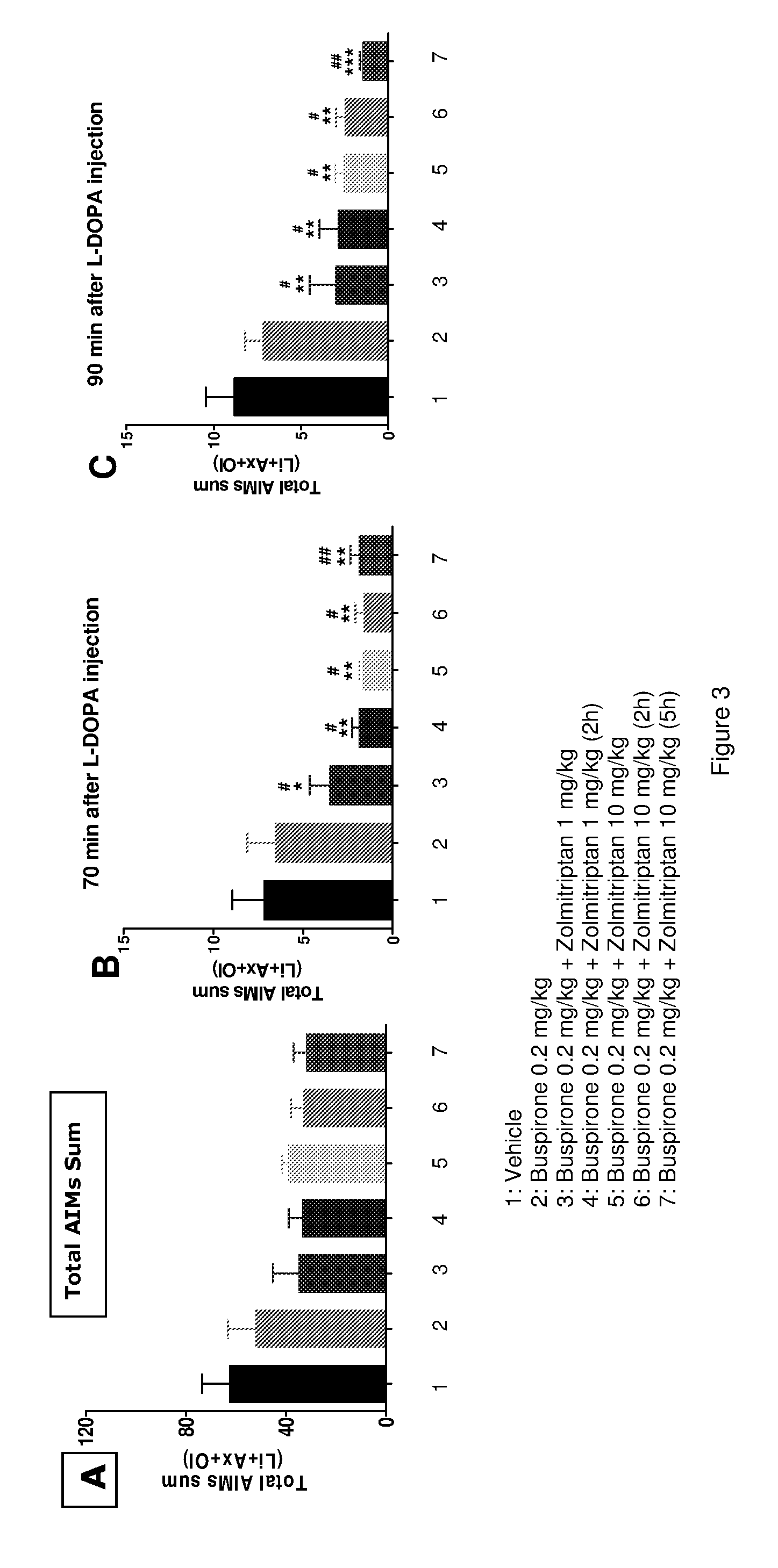
The present invention provides a pharmaceutical formulation for oral administration comprising an agonist of two or more of the 5-HT1B, 5-HT1D and 5-HT1F receptors, such as a triptan, e.g. zolmitriptan, in a matrix constituent with extended release characteristics, and further comprising a 5-HT1A-R agonist, such as buspirone, in a constituent with immediate-release characteristics. The special formulation is particularly well-suited for use in the treatment of movement disorders by combining the two active ingredients in a manner that achieves synergy from both the combination per se and the special release parameters of the pharmaceutical formulation, allowing for ease of administration and reducing the risk of adverse effects of each of the two active ingredients.
Owner:CONTERA PHARMA APS
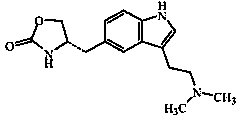
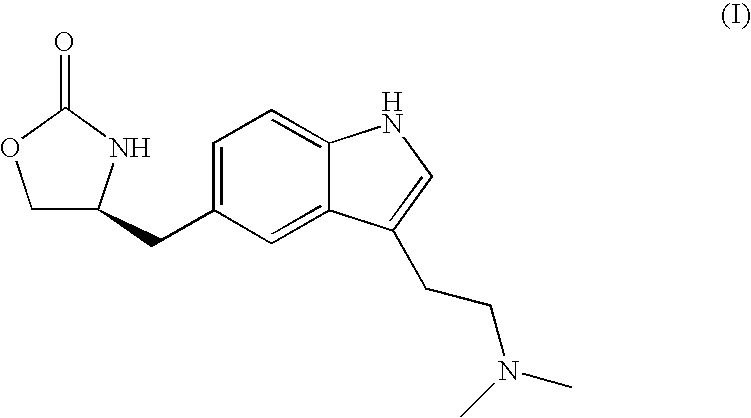
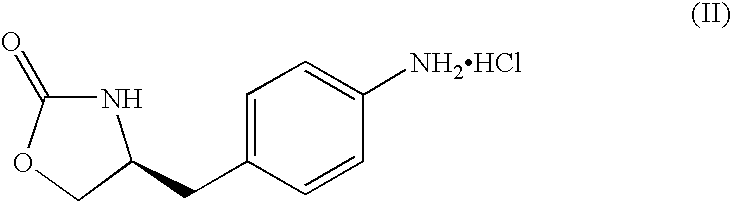
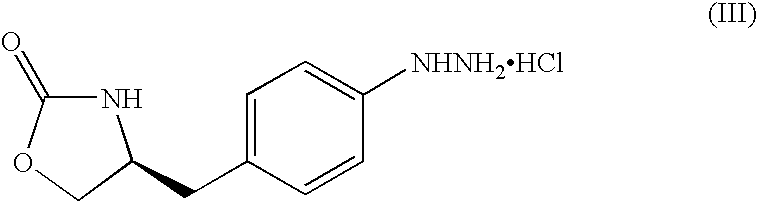
Zolmitriptan and preparation method thereof
ActiveCN103275075AImprove toleranceAdverse reactions are mild/moderateOrganic active ingredientsNervous disorderOrganic solvent1-Propanol
The invention relates to zolmitriptan and a preparation method thereof, in particular to the preparation method of zolmitriptan. The preparation method comprises the following steps: (1) reacting (S)-3-(4-nitrophenyl)-2-amino-1-propanol with triphosgene in an organic solvent in the presence of an alkaline reagent, and cyclizing to obtain (S)-4-(4-nitrobenzyl)-2-oxazolidinone; (2) carrying out hydrogenation reduction on (S)-4-(4-nitrobenzyl)-2-oxazolidinone in a solvent in the presence of a catalyst to obtain (S)-4-(4-aminobenzyl)-2-oxazolidinone; (3) carrying out diazo-reaction on (S)-4-(4-aminobenzyl)-2-oxazolidinone in an acidic solution in the presence of a diazotization reagent to obtain (S)-4-(4-hydrazinobenzyl)-2-oxazolidinone; and (4) reacting (S)-4-(4-hydrazinobenzyl)-2-oxazolidinone with 4-(N,N-dimethyl)-amino-dibutyl acetal to generate the zolmitriptan. The zolmitriptan prepared with the method is high in purity.
Owner:CHENGDU TIANTAISHAN PHARMA
Zolmitriptan powders for pulmonary delivery
The invention provides powder formulations containing zolmitriptan, or a pharmaceutically acceptable salt thereof, which are useful for pulmonary administration to the respiratory tract of a patient for the treatment of disease.
Owner:CIVITAS THERAPEUTICS
Orally disintegrating tablets of zolmitriptan and process for preparing the same
Silicon dioxide free orally disintegrating tablet formulations of zolmitriptan or a pharmaceutically acceptable salt thereof having magnesium carbonate heavy and sodium stearyl fumarate with one or more pharmaceutically acceptable excipients and a process for preparing such a formulation and its use in the treatment of migraines.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI
Zolmitriptan polymorphs
Crystalline polymorphic forms of zolmitriptan, solid amorphous zolmitriptan, and processes for preparing them.
Owner:DR REDDYS LAB LTD +1
Method for preparing Zolmitriptan
InactiveCN101693710AReduce manufacturing costSuitable for industrial productionOrganic chemistryState of artZolmitriptan
The invention relates to a method for preparing Zolmitriptan, which comprises the following steps: (1) obtaining a mixed solution containing (S)-4-(4-hydrazinobenzyl)-2-oxazolidinone by sequentially conducting diazotization reaction, reduction reaction and hydrolysis reaction on (S)-4-(4- aminobenzyl)-2-oxazolidinone; and (2) leading the (S)-4-(4-hydrazinobenzyl)-2-oxazolidinone in the mixed solution obtained in the step (1) and the dimethylamine butyral to carry out fisher-indole reaction so as to generate the Zolmitriptan, wherein in the step (2), the fisher-indole reaction is kept under the condition with the pH of 3-5. Compared with the prior art, the preparation method has the advantages that the molar yield is improved to 55%, the production cost of the Zolmitriptan is low and the preparation method is more applicable to industrial production.
Owner:SUZHOU LEADER CHEM
Process for the preparation of zolmitriptan, salts and solvates thereof
InactiveUS20110112157A1Minimize degradationHigh yieldBiocideNervous disorderCombinatorial chemistryPharmaceutical Substances
The present invention relates to an improved process for the preparation of the active pharmaceutical ingredient zolmitriptan. In particular, it relates to an efficient process for the preparation of zolmitriptan and its pharmaceutically acceptable salts and solvates.
Owner:GENERICS UK LTD
Method for the preparation of zolmitriptan
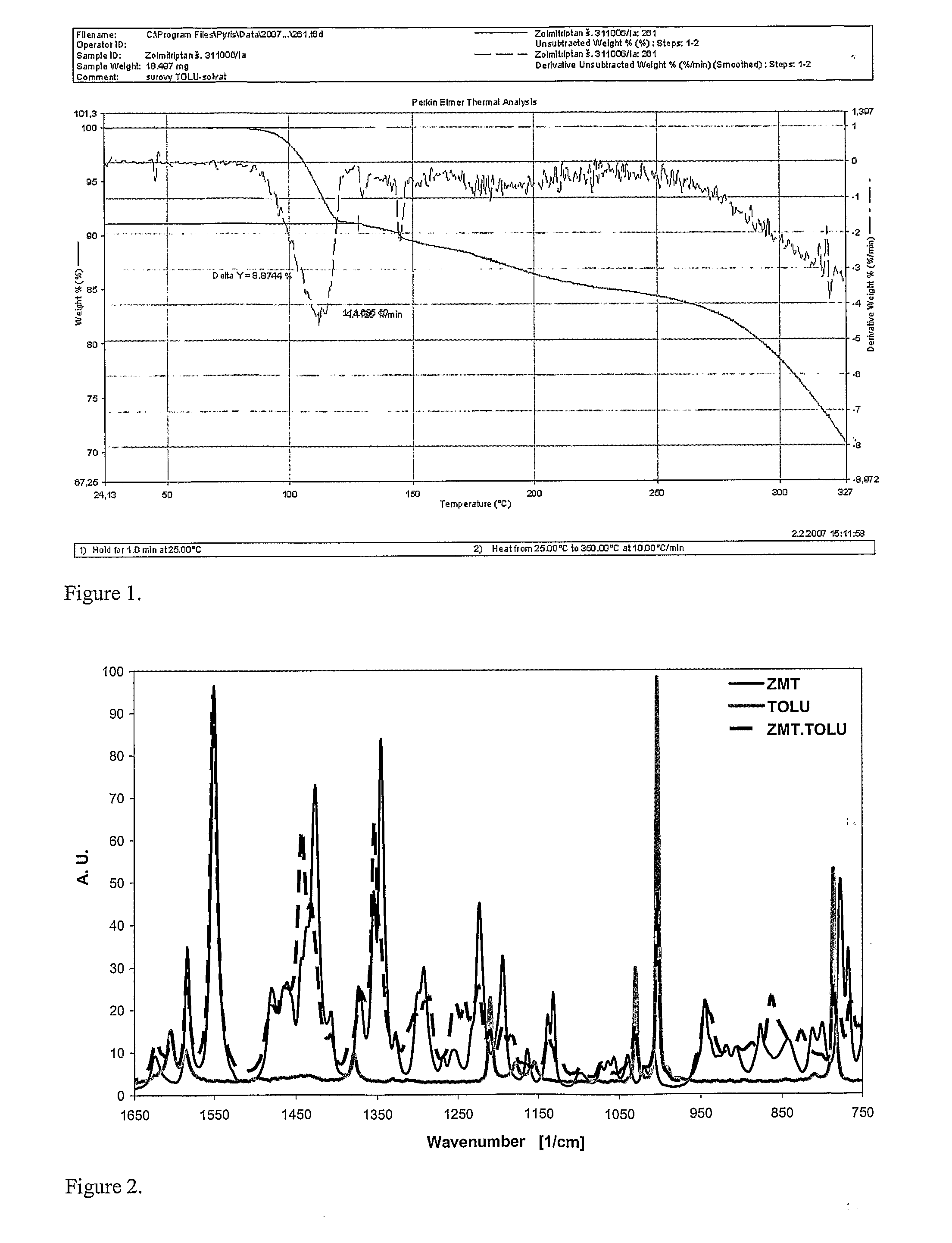
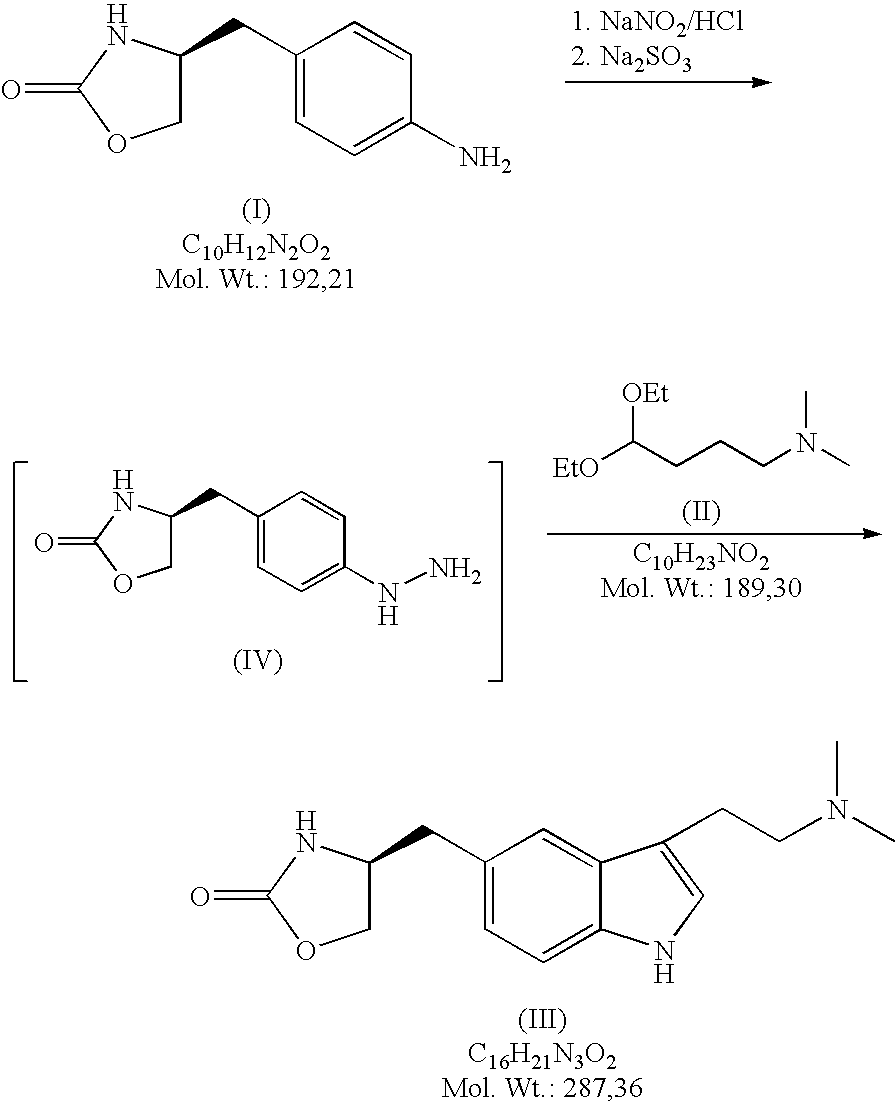
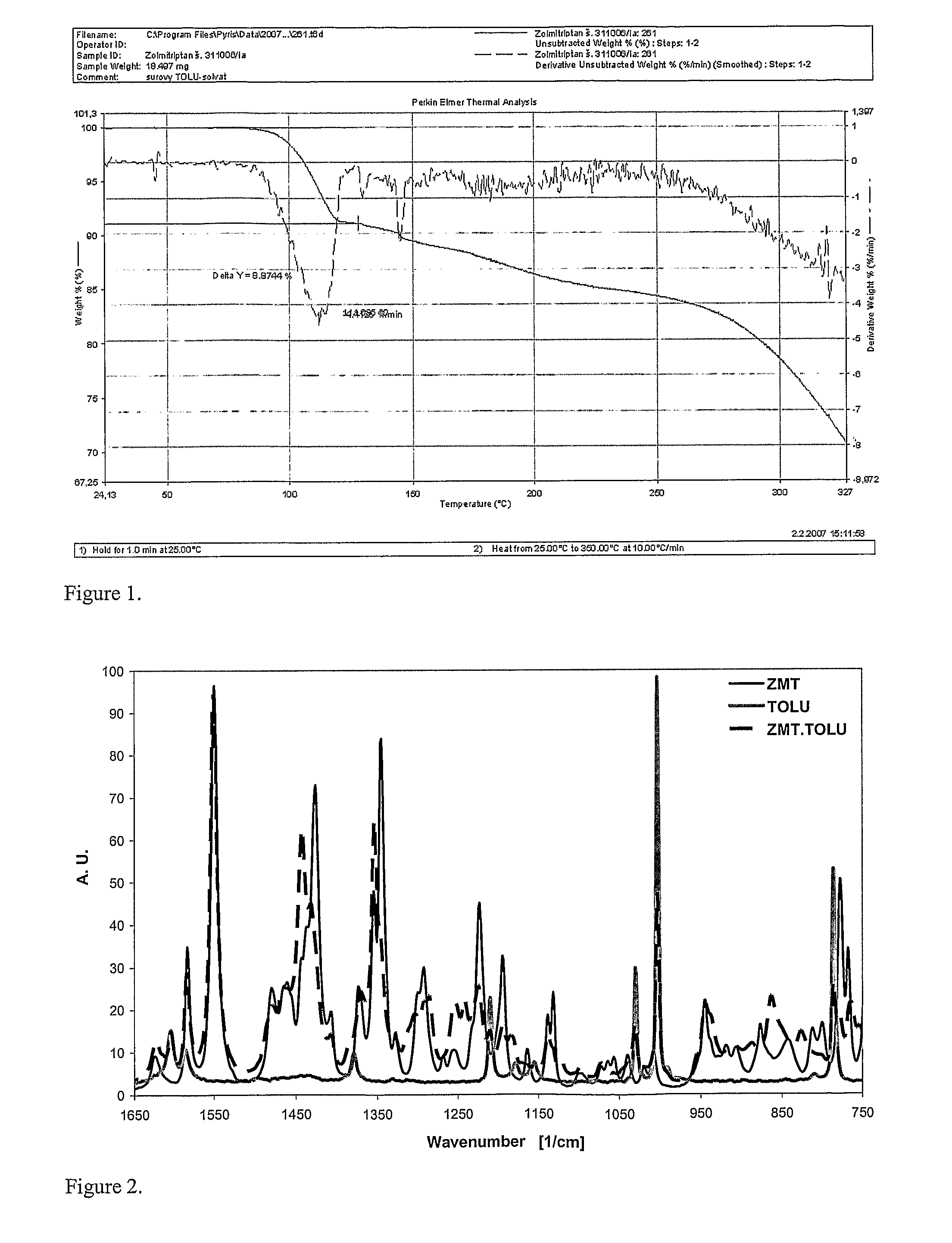
In the preparation of zolmitriptan of formula III the reduction of the diazonium salt to (5)-4-(4-hydrazinobenzyl)-1,3-oxazolidin-2-one of formula IV is performed in a more concentrated mixture and by the effect on an alkali metal disulphite, preferably sodium disulphite. A zolmitriptan toluene solvate, characterized by a toluene content of 9 to 14% by weight according to the gas chromatography determination and by a maximum of the corresponding mass loss at temperatures of about 111° C. in the gravimetric analysis record. A zolmitriptan toluene solvate, showing strong Raman bands at the wave numbers of 1443 and 1354 cm−1, characteristic for the crystal lattice of zolmitriptan with built-in toluene, and further marked bands at 1004 and 786 cm−1, characteristic for toluene.
Owner:ZENTIVA AS
Preparation method of zolmitriptan intermediate
ActiveCN110003129AMild reaction conditionsSimple post-processingOrganic chemistry methodsChemical recyclingOrganic synthesisReaction temperature
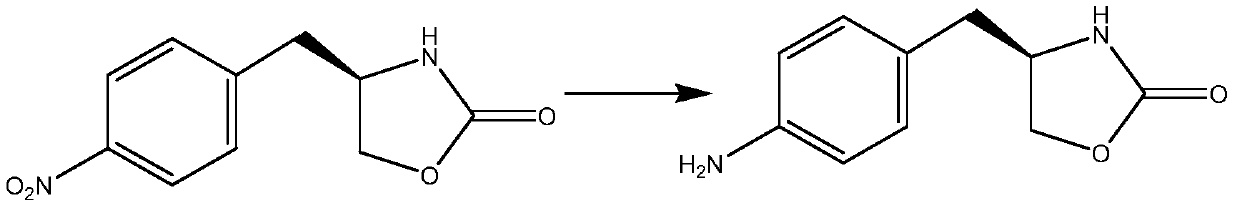
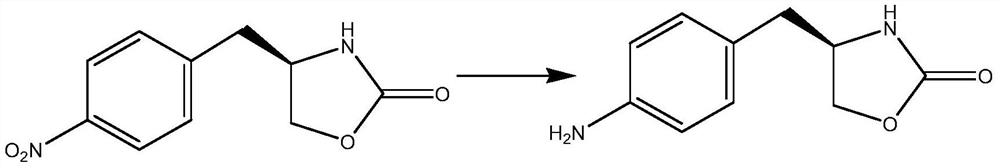
The invention belongs to the technical field of organic synthesis, and in particular relates to a preparation method of a zolmitriptan intermediate. (S)-4-(4-nitrobenzyl)-2-oxazolidinone used as a rawmaterial reacts with palladium-carbon and ammonium formate to prepare the zolmitriptan intermediate (S)-4-(4-aminobenzyl)-2-oxazolidinone. The method adopting the ammonium formate and palladium-carbon to carry out reduction has the advantages of low reaction temperature, simplicity in post-treatment, environmental friendliness, and recycling of a catalyst. The method allows the yield to reach 95%or more and the purity to reach 98% or more.
Owner:济南立德医药技术有限公司
Process for preparation of zolmitriptan
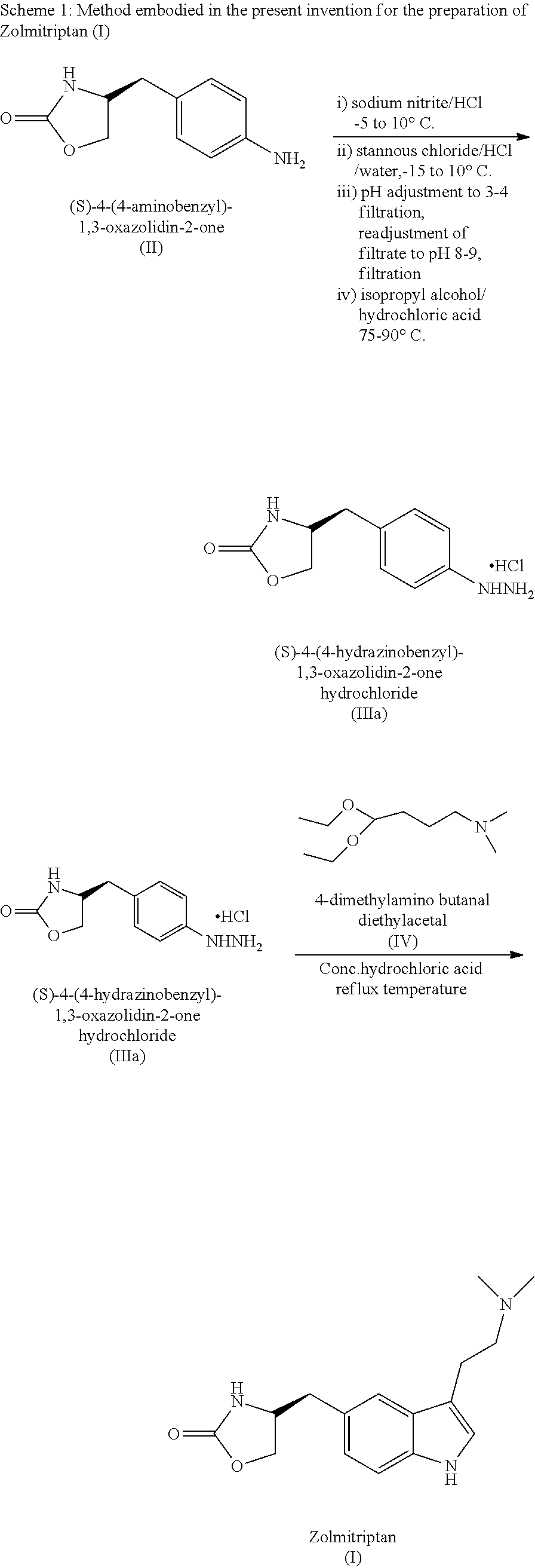
InactiveUS20140228582A1Cost-effective and convenient processOrganic chemistryTin(II) hydroxideZolmitriptan
The present invention provides a convenient and industrially viable process for preparation of Zolmitriptan (I) having desired purity. The invention specifically relates to a method for isolating (S)-4-(4-hydrazinobenzyl)-1,3-oxazolidin-2-one hydrochloride (IIIa) of desired purity by separating the undesired inorganic side products such as stannous hydroxide by manipulation of pH at different stages and finally treating with N,N-dimethylamino butyraldehyde diethyl acetal in an acidic medium to provide Zolmitriptan (I) conforming to regulatory specifications.
Owner:EMCURE PHARAMACEUTICALS LTD
A kind of synthetic method of carfilzomib
ActiveCN103641890BHigh reaction yieldHigh yieldPeptidesBulk chemical productionAcetic acidMorpholine
The invention relates to the technical field of drug synthesis, and particularly relates to a synthetic method of kyprolis. The synthetic method comprises the following steps: carrying out condensation reaction on morpholine-4-base acetic acid, L-homophenylalanine ester and salt, and then carrying out decarboxylation protection to generate a compound V; carrying out the condensation reaction on the decarboxylation, the salt and N-Boc-L-leucine, and then carrying out deamination protection to generate a compound VI; carrying out the condensation reaction on the compound V and the compound VI, and then carrying out decarboxylation protection to generate a compound VII; carrying out the condensation reaction on the compound VII and a compound VIII to obtain the kyprolis. The method disclosed by the invention can be used for enhancing the reaction yield by adopting a converging synthetic method; the used reagent is easy, convenient, easy to obtain and less in pollution. The process disclosed by the invention only relates to the condensation and deprotection between amino acids and is simple and controllable in reaction and suitable for industrial production.
Owner:CHONGQING SINTAHO PHARM CO LTD
A kind of preparation method of carfilzomib intermediate compound
ActiveCN105294501BMild conditionsSignificant technological progressCarbamic acid derivatives preparationOrganic compound preparationTert-Butyloxycarbonyl protecting groupCarfilzomib
Owner:SHANGHAI INST OF TECH
Amide boronic acid ester for detecting the purity of bortezomib intermediate, preparation method and application thereof
ActiveCN103965231BQuick checkEasy to operateComponent separationGroup 3/13 element organic compoundsChemical reactionFluorescence
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Enantioselective process for the preparation of zolmitriptan
An enantioselective process for preparing zolmitriptan, (S)-4-{[3-[2-(dimethylamine)ethyl]-1H-indol-5-yl]methyl}-2-oxazolidinone), by asymmetric hydrogenation of (Z)-2-(acetylamino)-3-{[3-N,N-(dimethylamine)ethyl)-1H-indol-5-yl]-2-propenoic acid methyl ester in the presence of hydrogen and an enantioselective chiral phosphine transition metal catalyst.
Owner:CHIRAL QUEST
Process for preparation of zolmitriptan
InactiveUS9006453B2Cost-effective and convenient processOrganic chemistryTin(II) hydroxideZolmitriptan
The present invention provides a convenient and industrially viable process for preparation of Zolmitriptan (I) having desired purity. The invention specifically relates to a method for isolating (S)-4-(4-hydrazinobenzyl)-1,3-oxazolidin-2-one hydrochloride (IIIa) of desired purity by separating the undesired inorganic side products such as stannous hydroxide by manipulation of pH at different stages and finally treating with N,N-dimethylamino butyraldehyde diethyl acetal in an acidic medium to provide Zolmitriptan (I) conforming to regulatory specifications.
Owner:EMCURE PHARAMACEUTICALS LTD
Zolmitriptan fast-release tablet and preparation method thereof
InactiveCN103751135AEasy to makeRelease quickly and completelyOrganic active ingredientsPill deliverySucroseFatty alcohol
The invention provides a zolmitriptan fast-release tablet, comprising the active component zolmitriptan, one or more selected from the group consisting of a filler, a disintegrating agent, a binder, a lubricant, a flow aid, a flavoring agent, a smell adjusting agent and a coloring agent, and a release promoter capable of promoting release of the active component. The release promoter may be selected from the group consisting of sodium dodecyl sulfate, poloxamer, Tweens, brominated cetane trimethylamine, sodium lauryl sulfate, stearyl alcohol sulfonate, polyoxyethylene high-grade fatty alcohol, sucrose ester, sorbitol fatty ester, soyabean lecithin, alginic acid, sodium alginate and colloid aluminium-magnesium silicate; and the usage amount of the release promoter is 0.1 to 5% of the total weight of the tablet.
Owner:SHANGHAI TIANLONG PHARMA
A kind of preparation method of zolmitriptan intermediate
ActiveCN110003129BMild reaction conditionsSimple post-processingOrganic chemistry methodsChemical recyclingPtru catalystOrganic synthesis
The invention belongs to the technical field of organic synthesis, and in particular relates to a preparation method of a zolmitriptan intermediate. The present invention takes (S)-4-(4-nitrobenzyl)-2-oxazolidinone as raw material, adopts palladium carbon and ammonium formate to react with it, and prepares zolmitriptan intermediate (S)- 4‑(4‑aminobenzyl)‑2‑oxazolidinone. The invention adopts ammonium formate and palladium charcoal for reduction, has low reaction temperature, simple post-treatment, is environmentally friendly, and the catalyst can be recycled and used mechanically. Adopting the present invention, the yield is above 95%, and the purity is above 98%.
Owner:济南立德医药技术有限公司
Method for the preparation of zolmitriptan
In the preparation of zolmitriptan of formula III the reduction of the diazonium salt to (5)-4-(4-hydrazinobenzyl)-1,3-oxazolidin-2-one of formula IV is performed in a more concentrated mixture and by the effect on an alkali metal disulphite, preferably sodium disulphite. A zolmitriptan toluene solvate, characterized by a toluene content of 9 to 14% by weight according to the gas chromatography determination and by a maximum of the corresponding mass loss at temperatures of about 111° C. in the gravimetric analysis record. A zolmitriptan toluene solvate, showing strong Raman bands at the wave numbers of 1443 and 1354 cm−1, characteristic for the crystal lattice of zolmitriptan with built-in toluene, and further marked bands at 1004 and 786 cm−1, characteristic for toluene.
Owner:ZENTIVA AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com