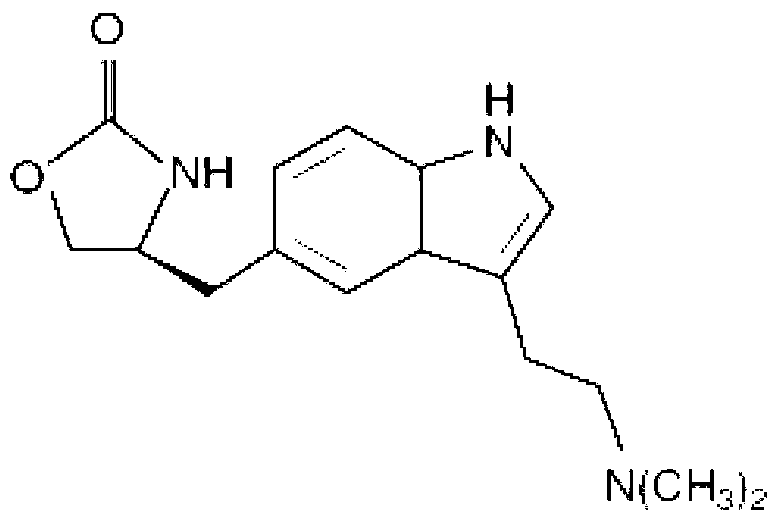
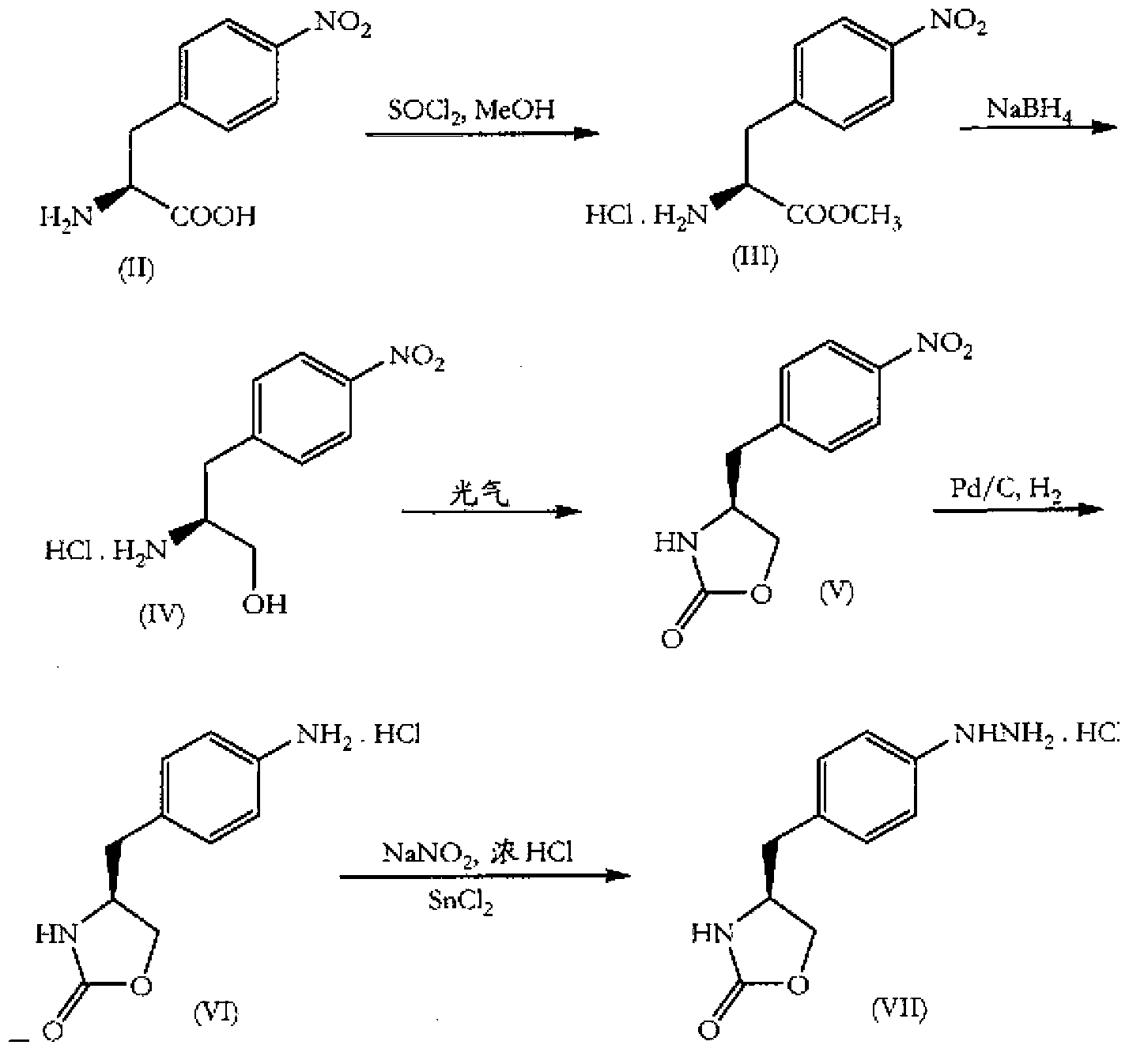
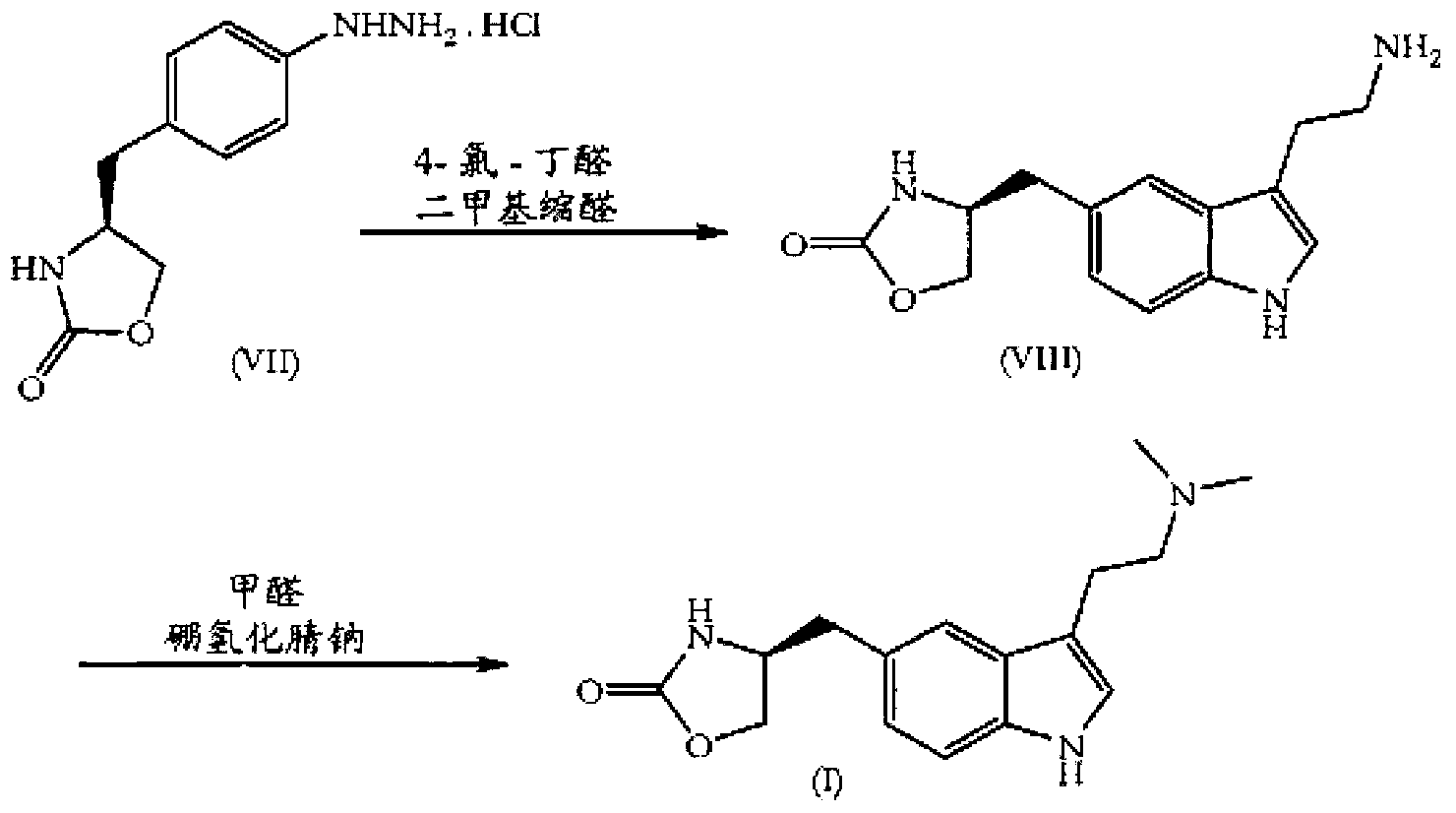
Zolmitriptan and preparation method thereof
A technology of oxazolidinone and reaction, which is applied in the field of pharmaceutical preparations and can solve problems such as lengthiness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Embodiment 1: the preparation of L-4-nitro-phenylalanine
[0105] Reaction formula:
[0106] Feeding ratio: L-phenylalanine 1kg, concentrated sulfuric acid 1700ml, concentrated nitric acid 450ml
[0107] Operation: In a reaction vessel equipped with a thermometer, add concentrated sulfuric acid according to the ratio, cool in an ice-salt bath to below 0°C and start to slowly add concentrated nitric acid dropwise. During the dropping process, control the temperature below 2°C; after dropping, control the temperature below 20°C Add L-phenylalanine in batches; after the addition, remove the ice-salt bath and let it rise to room temperature naturally, and stir for 30 minutes. Pour the reaction solution into 7L of ice water, control the temperature below 5°C while stirring, adjust the pH to 9.5-10.5 with 25% ammonia water, cool and crystallize, filter, and collect the solid; add 14L of hot water to heat and stir to dissolve the solid, and use the amount of feed Stir an...
Embodiment 2
[0108] Embodiment 2: the preparation of L-4-nitrophenylalanine methyl ester
[0109] Reaction formula:
[0110] Feed ratio: L-4-nitro-phenylalanine 720g, thionyl chloride 858ml, anhydrous methanol 3430ml
[0111] Operation: In a reaction vessel equipped with a thermometer, add anhydrous methanol according to the ratio, cool in an ice-salt bath to below 0°C and add thionyl chloride dropwise; after dropping, control the temperature below 2°C and stir for 30 minutes, then add L- 4-Nitro-phenylalanine was stirred in an ice-salt bath for 8 hours, removed from the ice bath, allowed to rise to room temperature naturally, and stirred for reaction. TLC monitors the reaction process (developing agent: chloroform: methanol = 1: 2), after the completion of the reaction (R f =0.8~0.85), use a rotary evaporator (control temperature 85±5°C) to concentrate under reduced pressure until no liquid drops out, add 750ml ice water and 1500mL ethyl acetate, and use saturated Na 2 CO 3 Soluti...
Embodiment 3
[0112] Embodiment 3: Preparation of (S)-3-(4-nitrophenyl)-2-amino-1-propanol
[0113] Reaction formula:
[0114] Feeding ratio: L-4-nitrophenylalanine methyl ester 200g, sodium borohydride 130g, methanol 2000ml, purified water 1000ml
[0115] Operation: In a reaction vessel equipped with a thermometer, add methanol and purified water according to the ratio, cool in an ice-salt bath to below 0°C, and add sodium borohydride in batches (control the reaction temperature not to exceed 2°C). After the addition is completed, add L-4-nitrophenylalanine methyl ester in batches (control the reaction temperature not to exceed 5°C). After the addition, stir and react at 0-5°C for 0.5 hours; then slowly rise to room temperature (about 20°C) , stirred for 3-5 hours; heated in a water bath to 50°C for 2-3 hours, TLC showed that the reaction was complete (developing solvent: dichloromethane: methanol = 2:1). Add 5% activated carbon to the reaction solution, decolorize at 50°C for 20 min...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com