A kind of preparation method of carfilzomib intermediate compound
A carfilzomib and compound technology, which is applied in the preparation of organic compounds, chemical instruments and methods, and the preparation of carbamic acid derivatives, can solve the problems of high process requirements, high cost, and low yield, and achieve simple experimental operation Easy to control, mild conditions, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Preparation of Compound IV
[0056]
[0057] In a 50mL three-necked flask equipped with a thermometer, a constant pressure titration funnel, and a glass stopper, add compound L-leucine Ⅴ (5.0g, 0.038mol), add NaOH (3.0g, 0.075mol) and 15mL of water, and vigorously Stir for 5 minutes to dissolve the material completely. Slowly add (Boc) dropwise at room temperature water bath temperature control 25~30℃ 2 O (10.8 g, 0.50 mol) in THF (5 mL) was slowly heated to 50-55°C after the dropwise addition. After 0.5h, a solution of NaOH (1.5g, 0.038mol) in water (5mL) was added to the system, and the reaction was incubated for 3h. After the reaction was complete by TLC, ethyl acetate (20mL) was added to the system. Under an ice-water bath, adjust the pH value to 2-3 with 6N dilute hydrochloric acid, filter the ethyl acetate phase, wash the water phase with ethyl acetate (20 mL), combine the ethyl acetate phases, and successively wash with saturated NaHCO 3 , saturat...
Embodiment 2
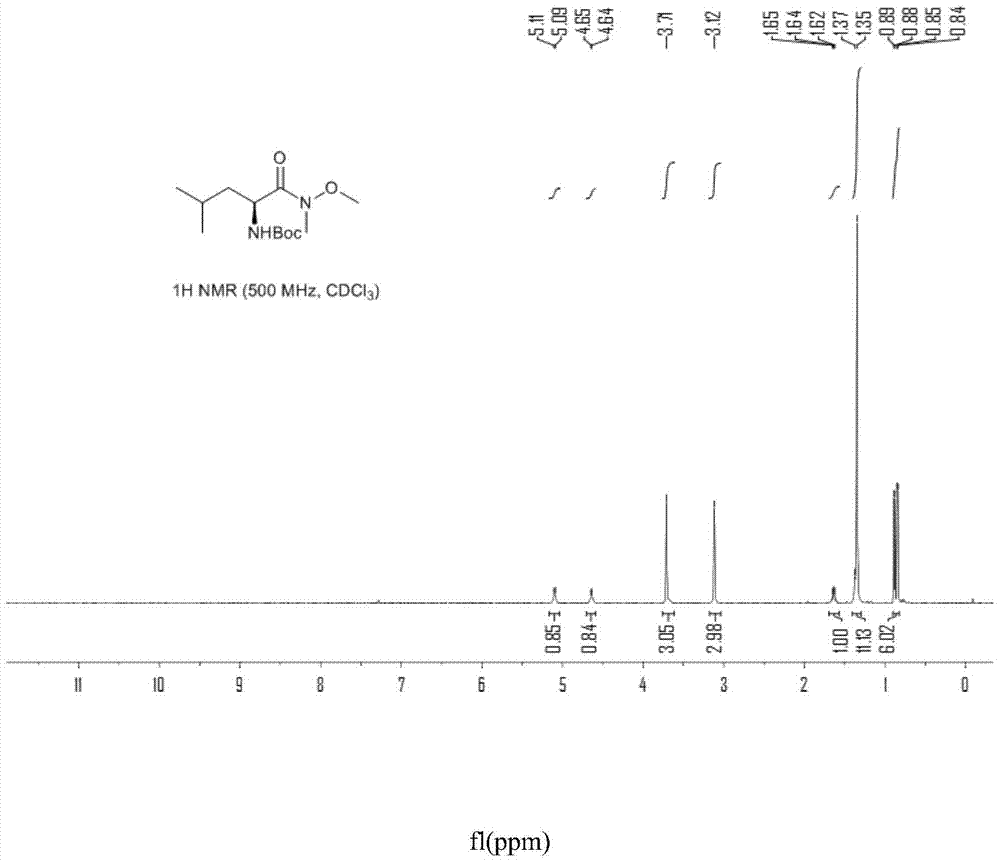
[0058] Example 2 Preparation of Compound III
[0059]
[0060] In a 100mL three-neck flask equipped with a thermometer, a constant pressure dropping funnel and a drying tube, add L-Boc-leucine Ⅳ (5.0g, 0.022mol) and dichloromethane (25mL), and stir vigorously for 5min to dissolve the material completely . CDI (5.6 g, 0.035 mol) was slowly added in batches under stirring in a room temperature water bath controlled at 25-30° C., and kept for 3 h. After no raw materials were detected by TLC, N,O-dimethylhydroxylamine hydrochloride (3.4 g, 0.035 mol) was slowly added in batches at 20-25° C., and reacted for 1 h. TLC detects that the intermediate state reaction is complete, and the organic phase is washed twice with 25mL water, once with 25mL 1N dilute hydrochloric acid, twice with 25mL saturated sodium bicarbonate solution, once with 50mL saturated saline, and then washed with Na 2 SO 4 After drying, the system solvent was distilled off under reduced pressure to obtain 6.42 ...
Embodiment 3
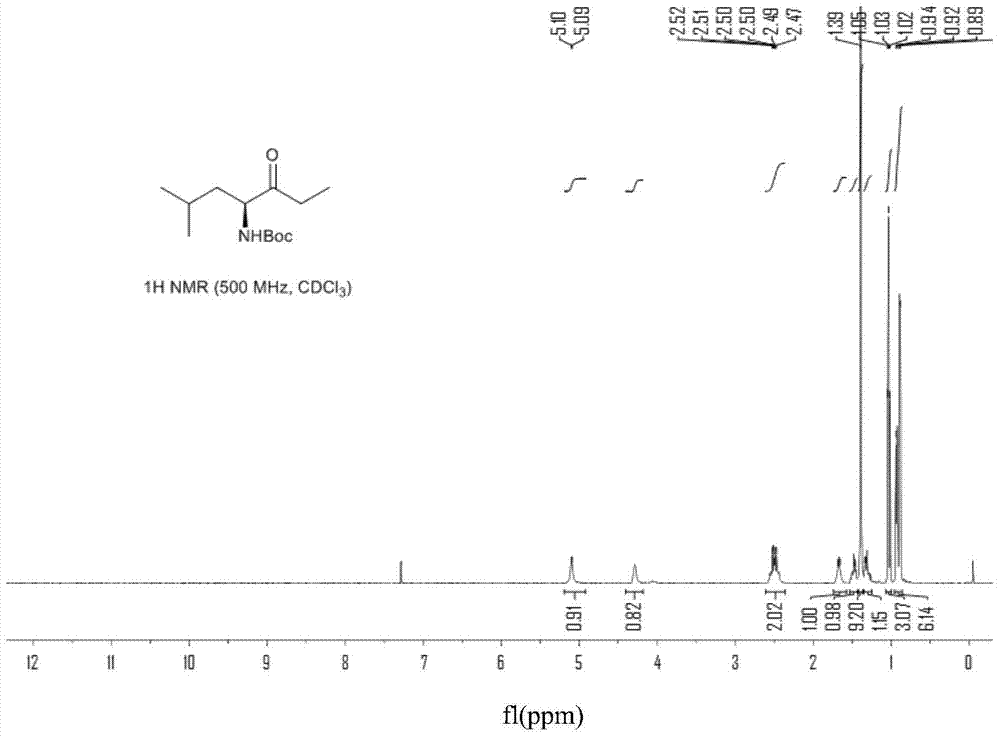
[0061] Example 3 Preparation of Compound II
[0062]
[0063] Add III (8.2g, 0.030mol) and 40mL THF to a 250mL three-neck flask equipped with a thermometer, constant pressure dropping funnel and nitrogen protection, and stir vigorously for 5min to dissolve the material completely. Under the condition of room temperature controlled by water bath at 20-25°C, slowly add THF solution of ethylmagnesium chloride (45mL, 0.090mol) dropwise, then maintain the temperature in the range of 25-30°C for 3-4h, TLC detection until the end of the reaction. Slowly add 100mL of 1N dilute hydrochloric acid to the system to quench the system at room temperature, and add 100mL of ethyl acetate to extract the product. The ethyl acetate phase is washed twice with 50mL of saturated sodium bicarbonate solution, and then washed with 50mL of saturated brine. 2 SO 4 After drying, the solvent in the system was evaporated under reduced pressure, and the crude product was subjected to column chromatograp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com