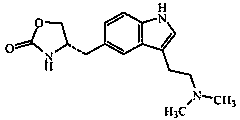
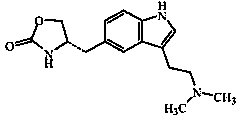
Lyophilized zolmitriptan nanometer powder and preparation method thereof
A technology of nano freeze-dried powder and zolmitriptan, which is applied in the field of pharmaceuticals, can solve the problems of high equipment requirements and difficulty in industrial production, achieve good biocompatibility, avoid the problem of organic solvent residue, and increase the degree of dispersion Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
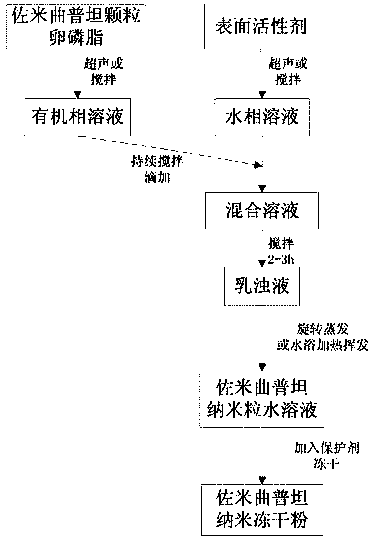
[0027] The preparation method of zolmitriptan nano freeze-dried powder provided by the invention comprises the following steps:
[0028] (1) Add zolmitriptan and carrier materials into an organic solvent, and dissolve by ultrasonication or stirring to obtain an organic phase solution A.
[0029] (2) Add the surfactant into purified water, stir until dissolved, and obtain aqueous phase solution B.
[0030] (3) Under the condition of stirring, the organic phase solution A is slowly added dropwise to the aqueous phase solution B to obtain a mixed solution C, and the mixed solution C is continuously stirred to obtain an emulsion D.
[0031] (4) Volatilize the emulsion D in a water bath at 50-60° C. to remove the organic solvent to obtain an aqueous solution of zolmitriptan nanoparticles.
[0032] (5) Adding a protective agent to the aqueous solution of zolmitriptan nanoparticles, and then freeze-drying to obtain zolmitriptan nanometer freeze-dried powder.
[0033] The step of fr...
Embodiment 1
[0040] The present embodiment prepares raw materials and other ingredients according to the following proportions by weight:
[0041]
[0042]
[0043] Dissolving the formula amount of zolmitriptan and lecithin in an appropriate amount of ethyl acetate, and ultrasonically dissolving it to obtain an organic phase solution A; adding the formula amount of Tween 80 into an appropriate amount of purified water, stirring to dissolve it to obtain an aqueous phase solution B; Under the condition of continuous stirring, the organic phase solution A is slowly added dropwise to the aqueous phase solution B, and the mixed solution C is obtained after the dropwise addition is completed; the mixed solution C is continuously stirred for 2.5 hours to obtain the emulsion D; the emulsion D The organic phase was volatilized and removed by continuous stirring in a water bath at 57° C. to obtain an aqueous solution of zolmitriptan nanoparticles.
[0044]After testing, the obtained zolmitript...
Embodiment 2
[0048] The present embodiment prepares raw materials and other ingredients according to the following proportions by weight:
[0049]
[0050] Dissolving the formula amount of zolmitriptan and lecithin in an appropriate amount of ethyl acetate, and ultrasonically dissolving it to obtain an organic phase solution A; adding the formula amount of Tween 80 into an appropriate amount of purified water, stirring to dissolve it to obtain an aqueous phase solution B; Under the condition of continuous stirring, the organic phase solution A is slowly added dropwise to the aqueous phase solution B, and the mixed solution C is obtained after the dropwise addition; the mixed solution C is continuously stirred for 2.3 hours to obtain the emulsion D; the emulsion D The organic phase was volatilized and removed by continuous stirring in a water bath at 57° C. to obtain an aqueous solution of zolmitriptan nanoparticles.
[0051] The obtained zolmitriptan nanoparticle aqueous solution has an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com