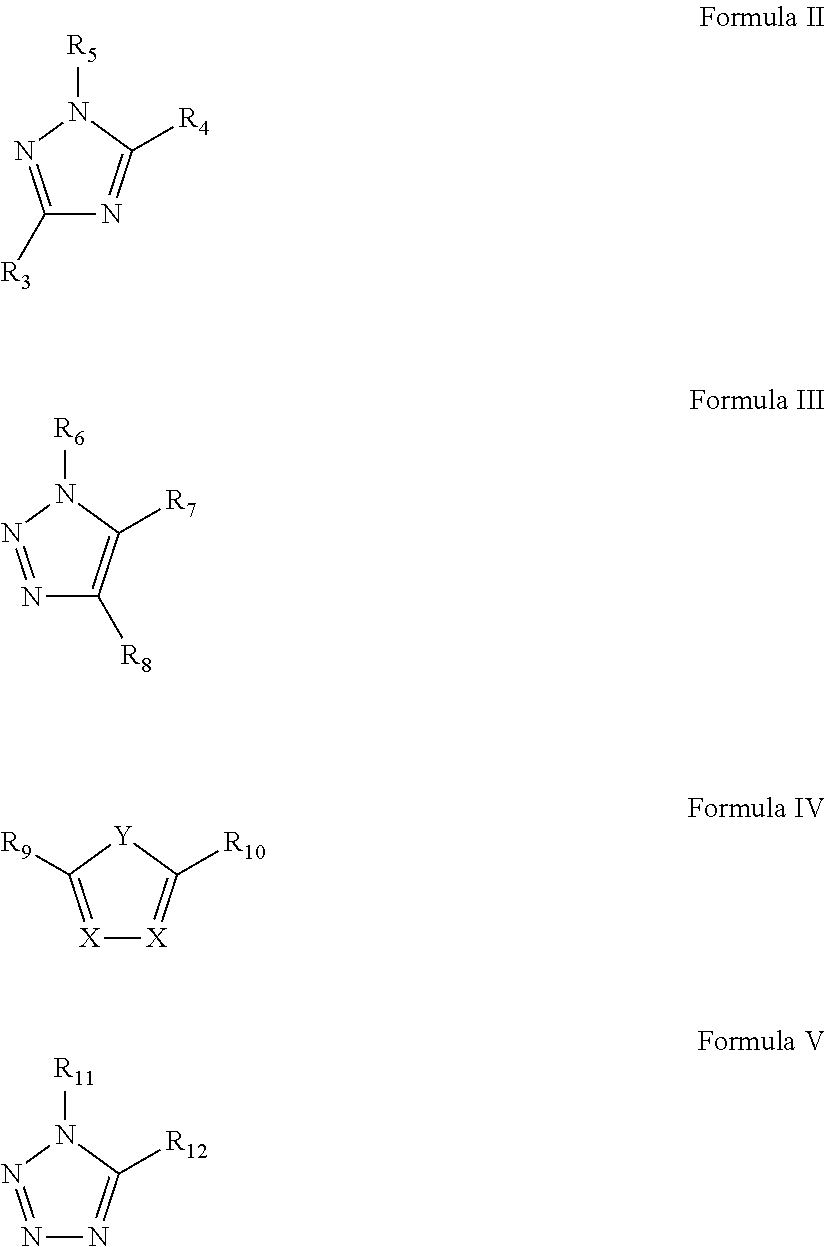
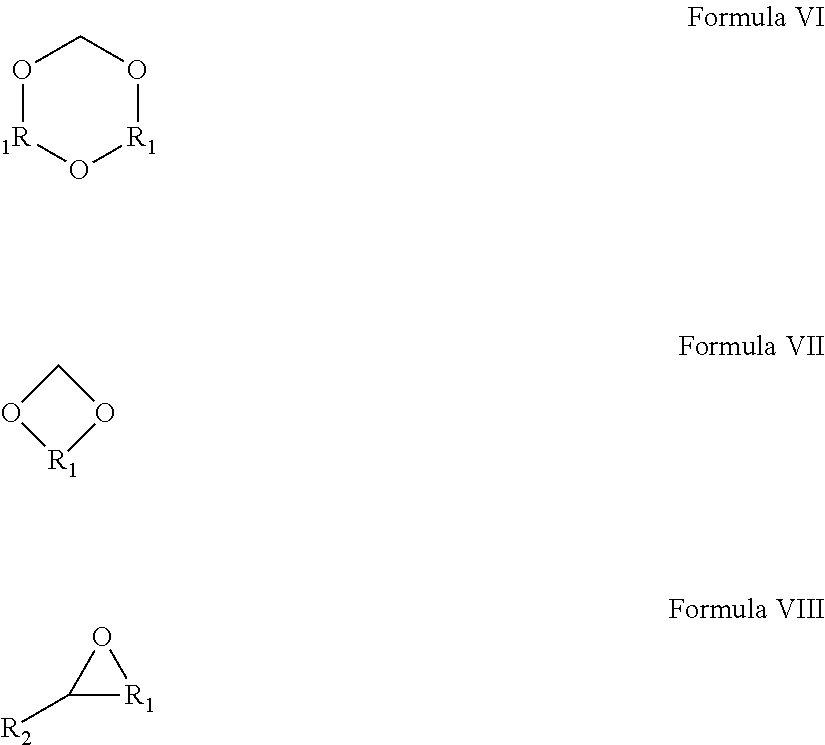
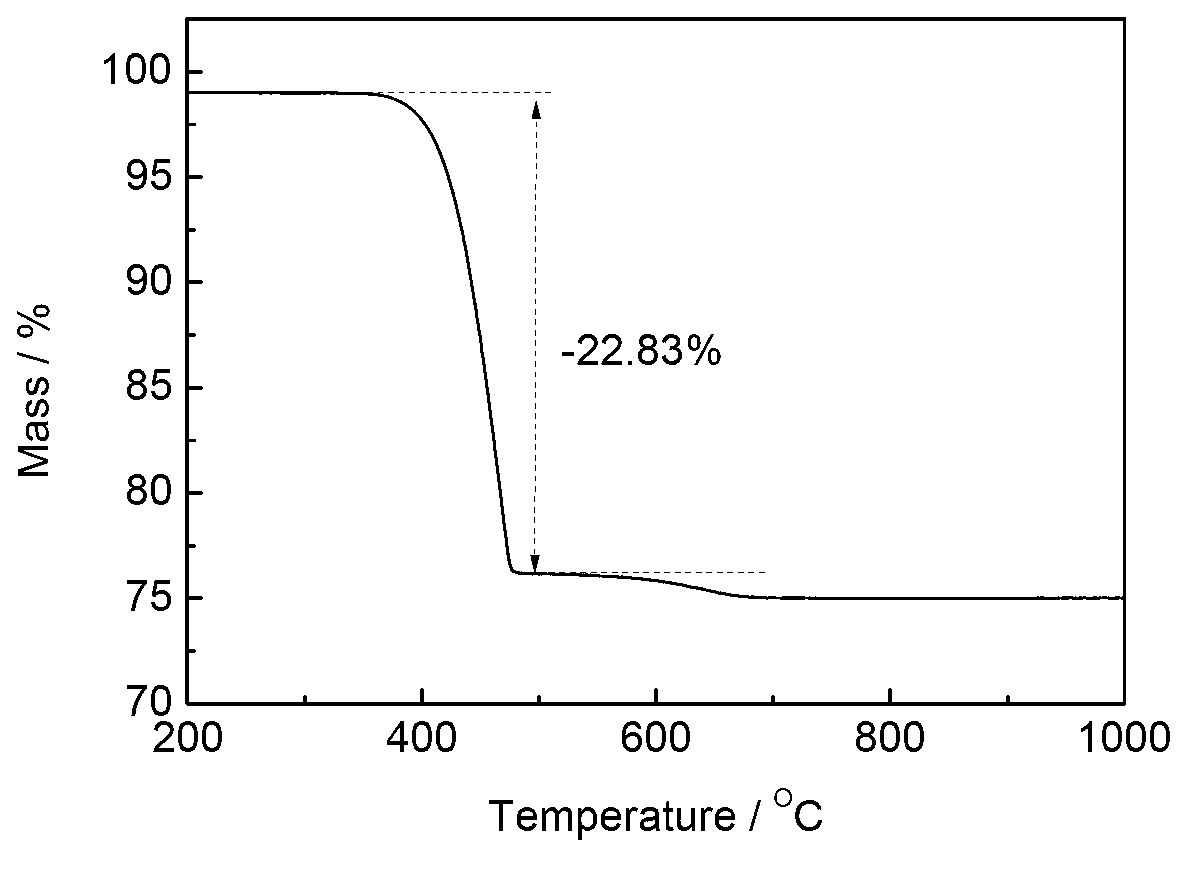
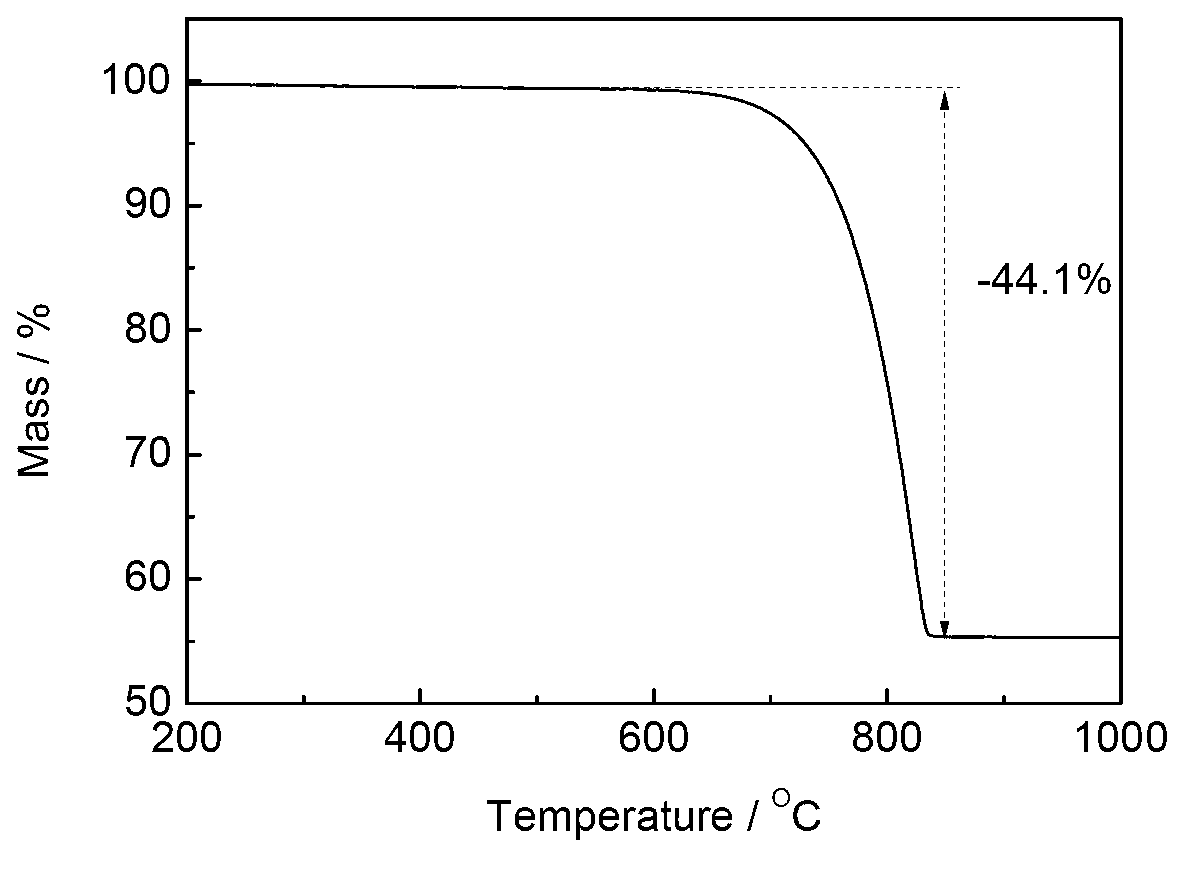
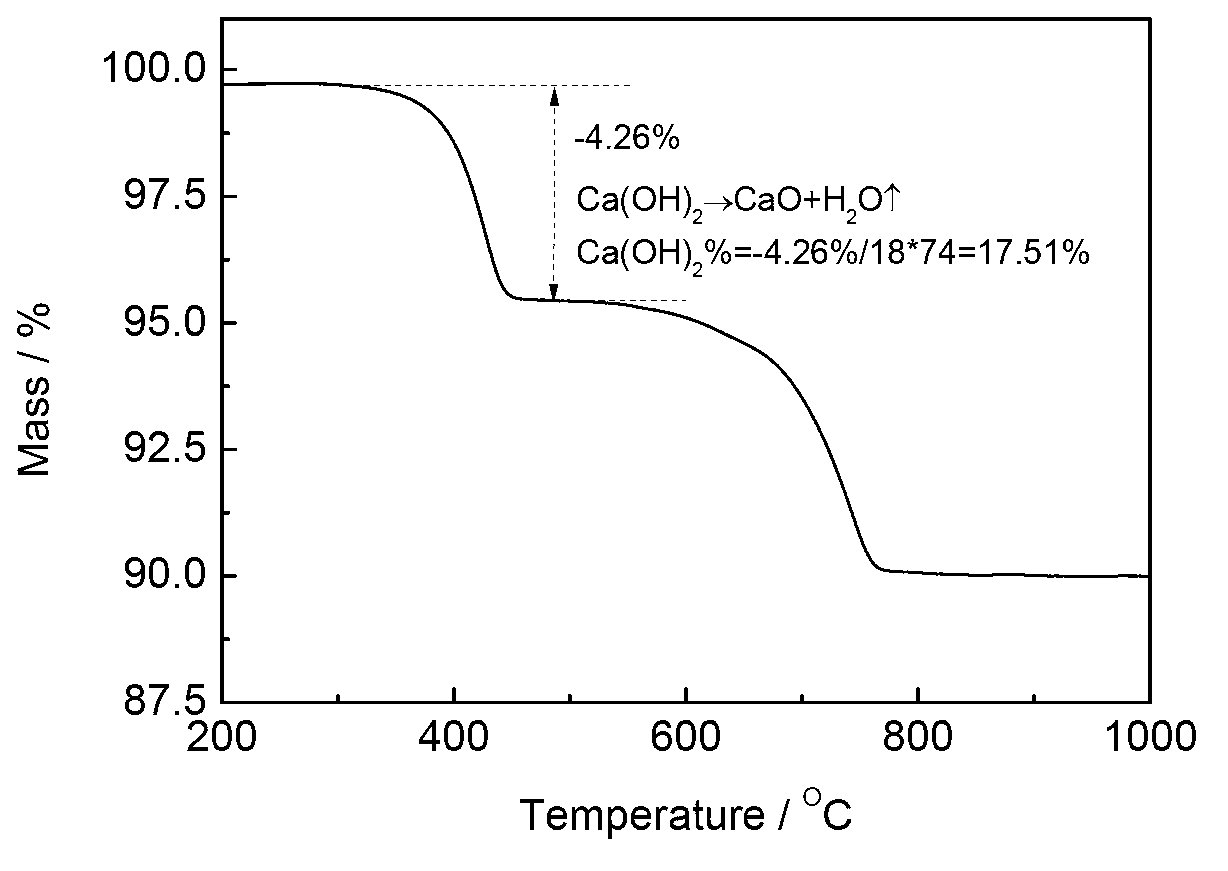
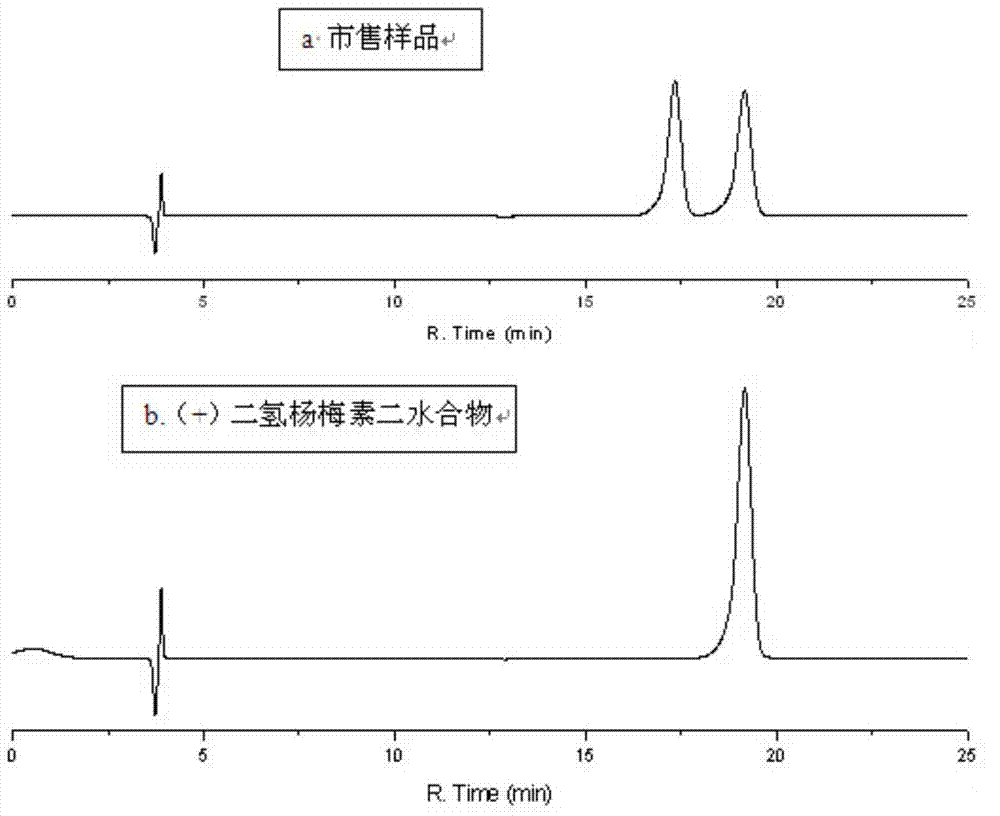
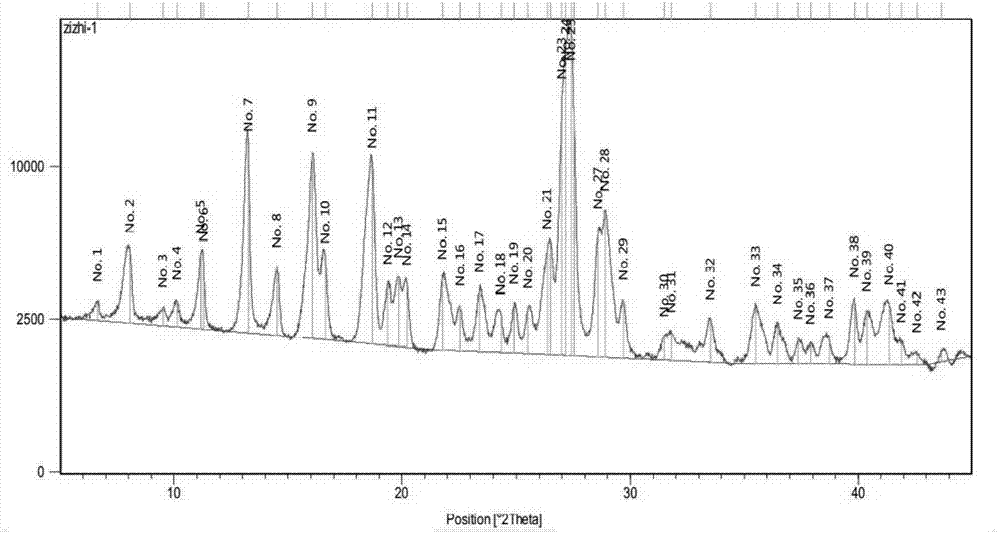
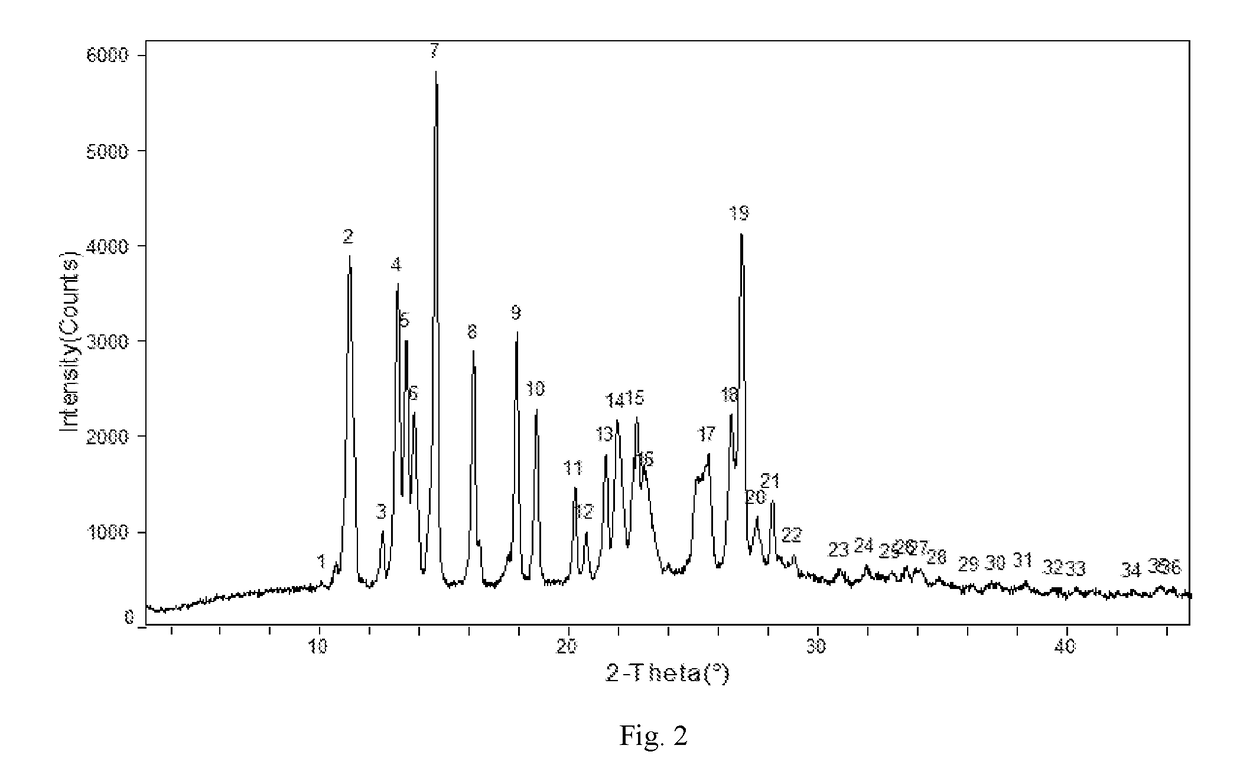
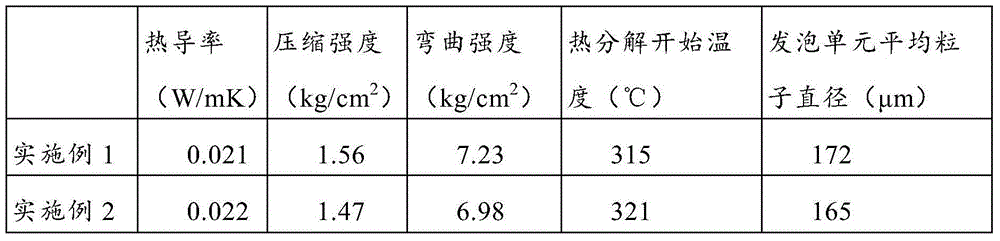
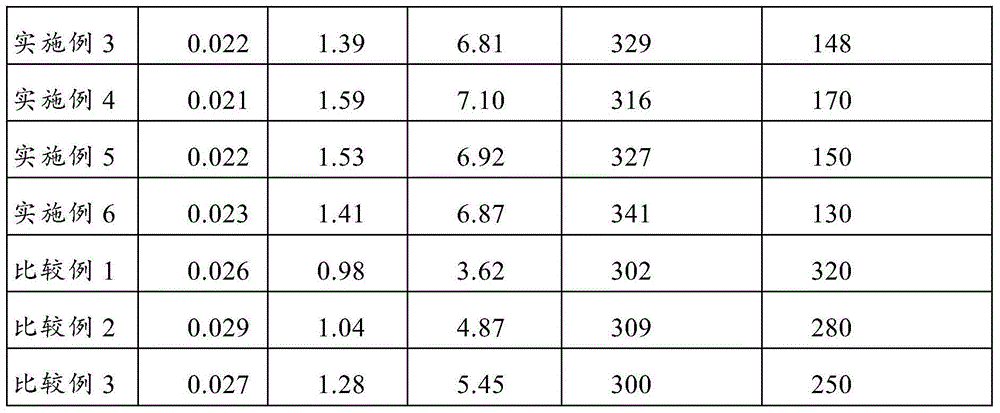
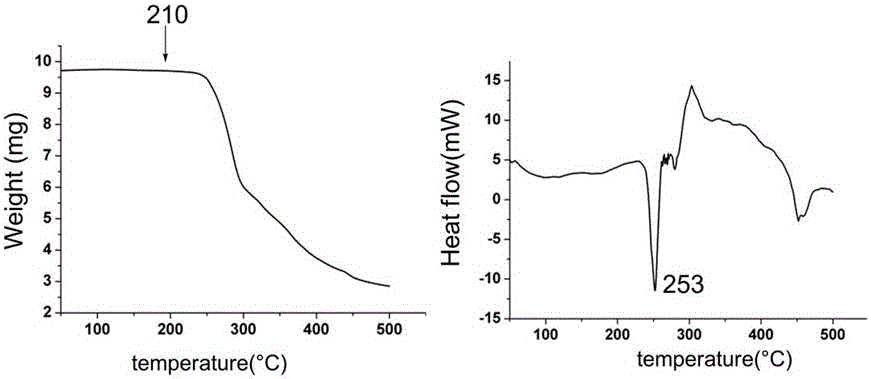
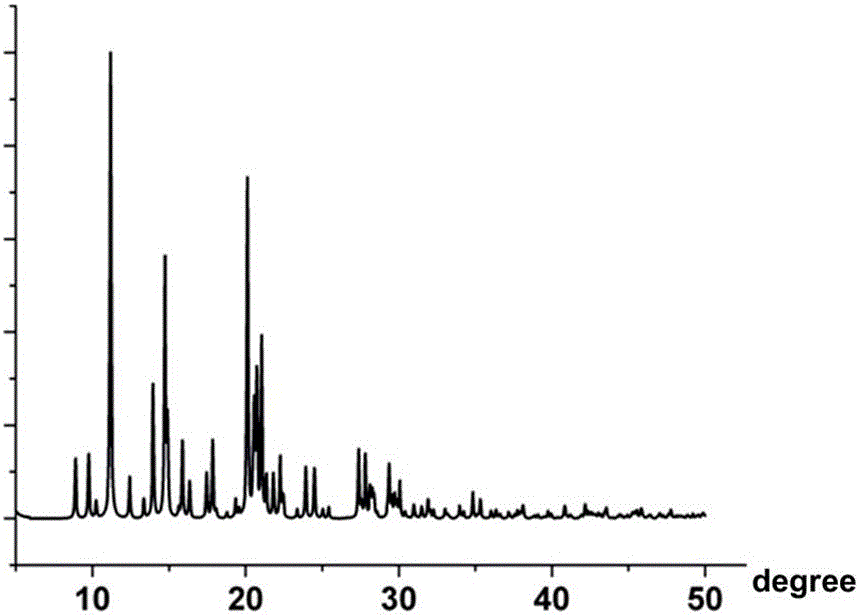
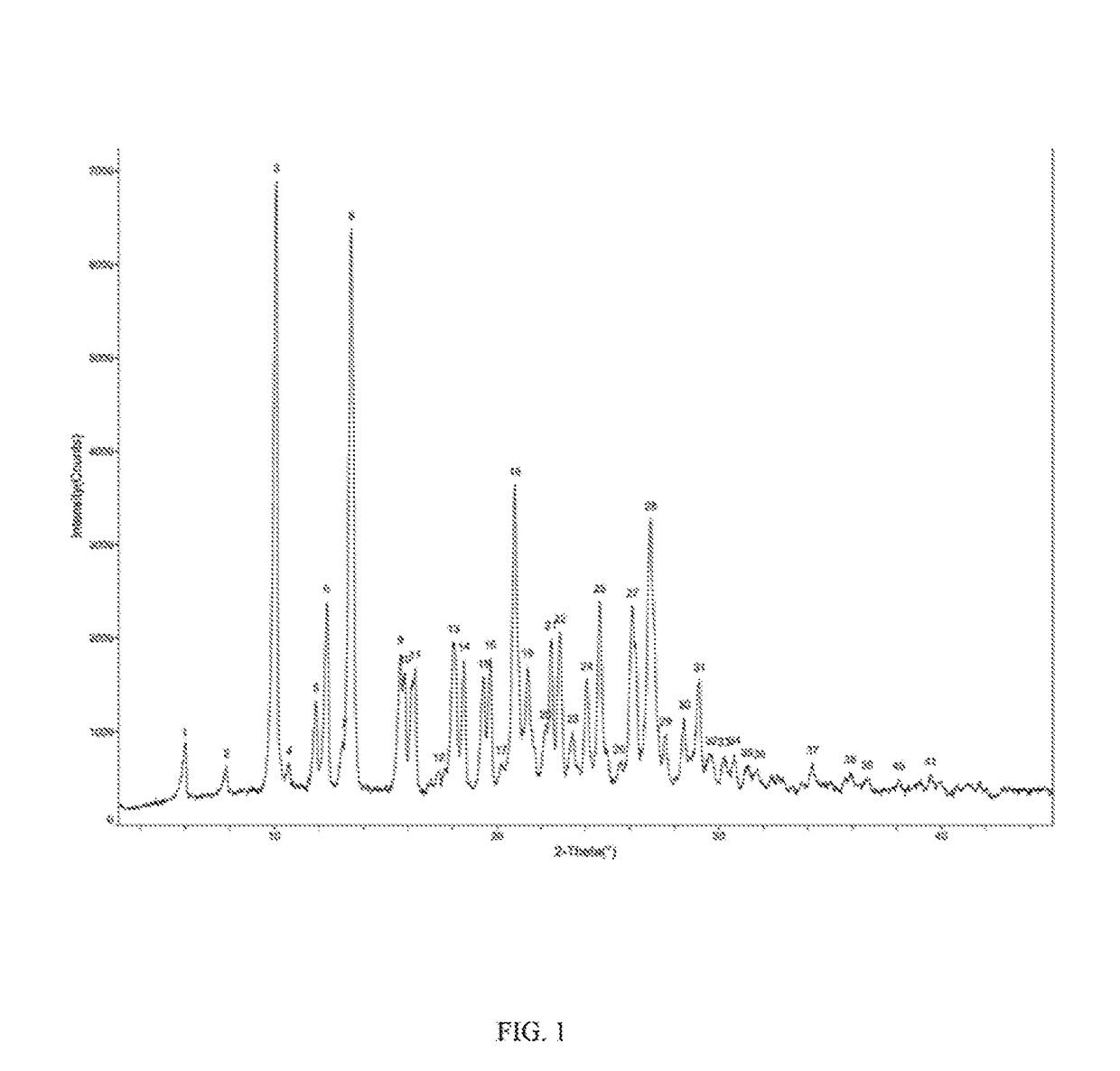
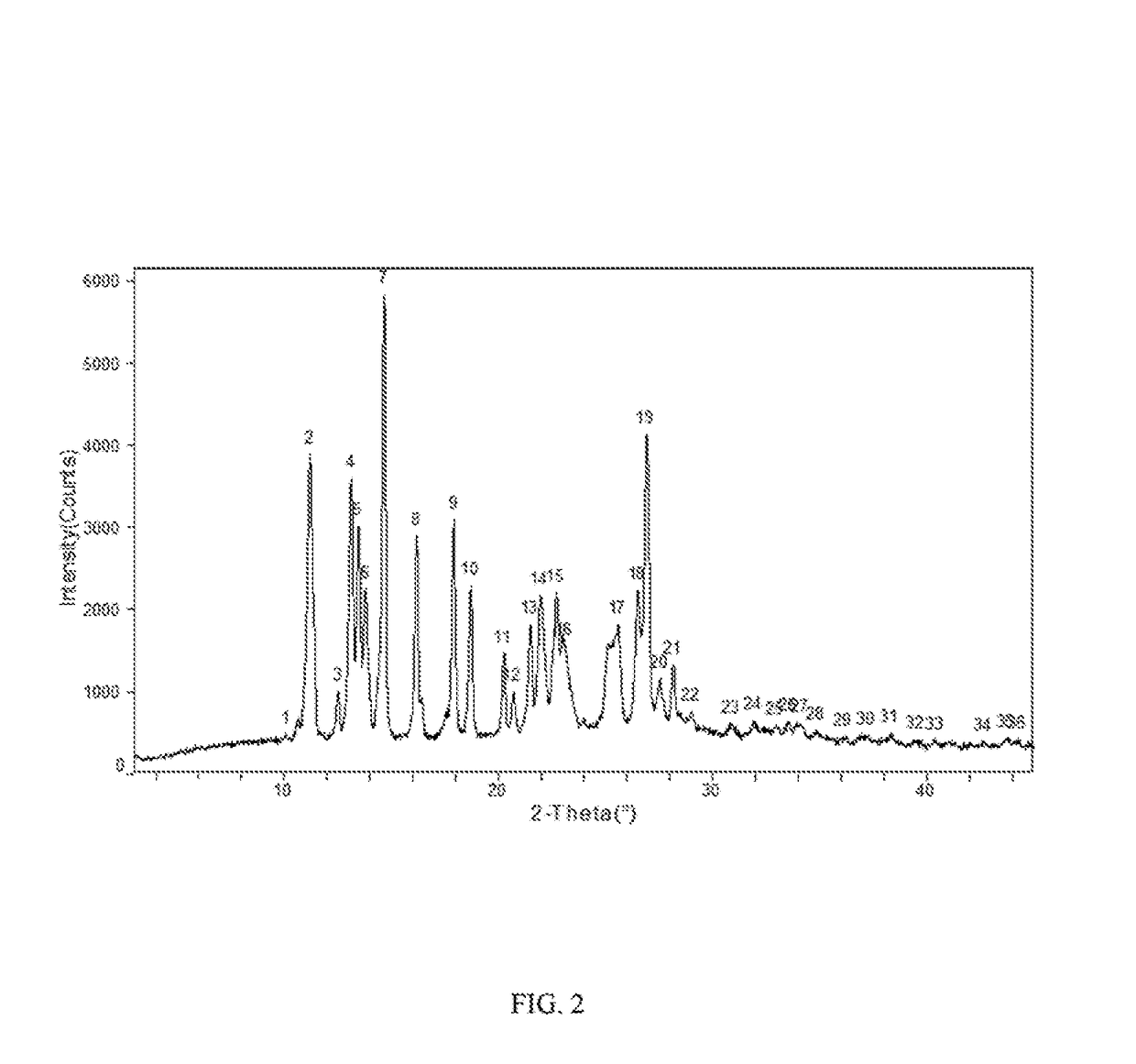
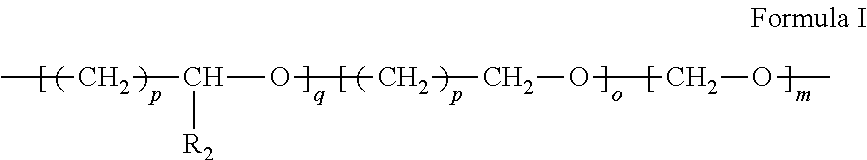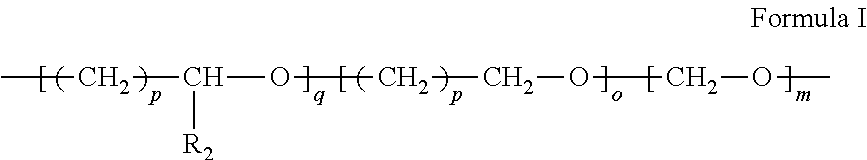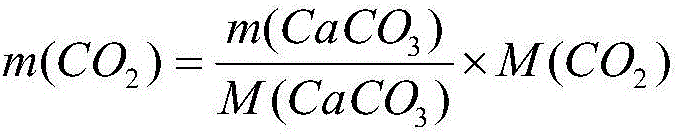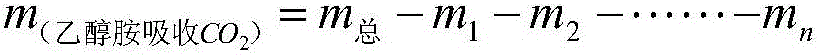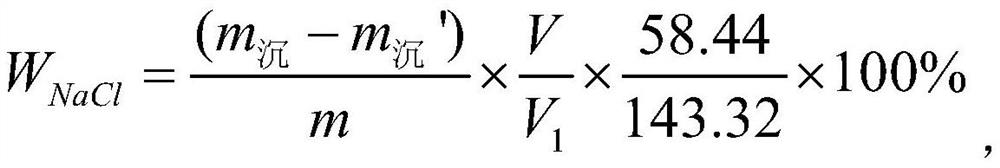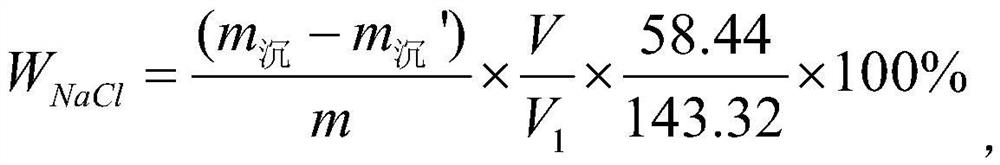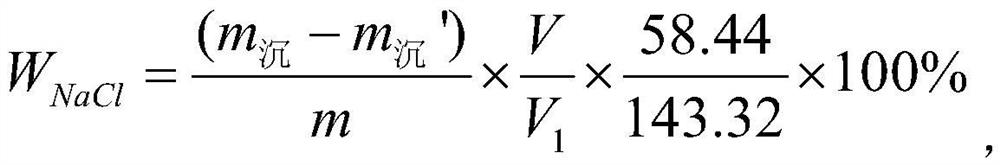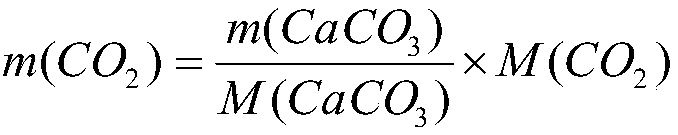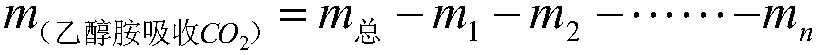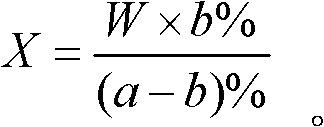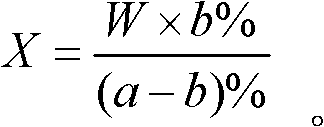Patents
Literature
37 results about "Gravimetric analysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte (the ion being analyzed) based on its mass. The principle behind this type of analysis is that once an ion's mass has been determined as a unique compound, that known measurement can then be used to determine the same analyte's mass in a mixture, as long as the relative quantities of the other constituents are known.
Dispersion comprising metallic, metal oxide or metal precursor nanoparticles
ActiveUS20140065387A1Stable and concentrated dispersionOrganic chemistryConductive materialDecompositionNanoparticle
The present invention relates to a dispersion comprising metallic, metal oxide or metal precursor nanoparticles and a polymeric dispersant, the dispersant comprising an anchor group with affinity for the metallic, metal oxide or metal precursor nanoparticles that is chemically bonded to a polymeric backbone characterized in that the dispersant has a 95 wt. % decomposition at a temperature below 300° C. as measured by Thermal Gravimetric Analysis. It further relates to metallic fluids or inks prepared from the dispersion and to the preparation of the dispersion and the metallic fluid or inks.
Owner:AGFA GEVAERT AG
Method for preparing tetrabasic lead sulfate by using acid leaching byproduct in production of lead acid storage battery positive electrode plate
InactiveCN104218270ASlow down early capacity fading phenomenonImprove cycle lifeWaste accumulators reclaimingLead sulfatesElectrical batteryLead oxide
The invention relates to a method for preparing tetrabasic lead sulfate by using an acid leaching byproduct in the production of a lead acid storage battery positive electrode plate. The method comprises the following steps: 1, draining water on the surface of the acid leaching byproduct, respectively determining the content of lead sulfate, lead oxide, simple substance lead and water in the acid leaching byproduct through a chemical analysis technology and a gravimetric analysis technology, and determining the percentage content of lead sulfate and lead oxide; 2, adding the surface water drained acid leaching byproduct into a reaction kettle, supplementing lead oxide or lead powder used for producing the lead acid storage battery according to the determination results obtained in step 1 to make a molar ratio of lead oxide to lead sulfate in the reaction kettle reach 3-6.5:1, and using deionized water as a reaction medium in a reaction system; and 3, preparing the tetrabasic lead sulfate. The above raw material is the lead acid battery byproduct and is applied to the production of the lead acid battery, so waste recycling is realized, the production cost of the lead acid storage battery is reduced, the method is environmentally friendly, and the quality of the battery is improved.
Owner:CHAOWEI POWER CO LTD
Method for accurately determining calcium-based desulfurization agent main component
ActiveCN103134736AAccurate measurementQuality improvementWeighing by removing componentChemical analysis using titrationCalcium hydroxideSucrose solution
The invention relates to a method for accurately determining calcium-based desulfurization agent main component. The method comprises the steps that: 1, a proper amount of a desulfurization agent sample is weighed, and is placed into a container bottle; a proper amount of a sucrose solution is added; the bottle is plugged and oscillated until completely decomposed; the material is filtered, such that a filtrate (1) and a precipitate (1) are obtained; 2, the precipitate (1) and the filtering paper are placed in a heating vessel; a proper amount of an acetic acid solution is added; the mixture is heated until the sample is completely dissolved; filtering is carried out, such that a filtrate (2) and a precipitate (2) are obtained; 3, the precipitate (2) and the filtering paper are added into the heating vessel; a proper amount of a mixed acid solution is added; the mixture is heated until the solution shows an earth yellow color; salt is dissolved, and heating is continued until the mixture is slightly boiling; the mixture is diluted and filtered, such that a filtrate (3) is obtained; 4, the filtrates are respectively transferred into volumetric flasks, and volumes are metered; two parts of proper amounts of the samples are fetched; and 5, combined with chemical analysis and thermal gravimetric analysis, true and valid calcium oxide content in the desulfurization agent can be determined, and the contents of calcium hydroxide and calcium carbonate which severely affecting desulfurization performance can be determined.
Owner:INST OF RES OF IRON & STEEL JIANGSU PROVINCE
Five different crystalline form substances of dihydromyricelin
The invention discloses five crystalline form substances of dihydromyricelin (of which the chemical name is 3,5,7,3',4',5'-hexahydroxy-2,3-dihydroflavonol and the English name is dihydromyricelin or ampelopsin). The spatial configuration of dihydromyricelin is characterized by virtue of colorimetry-polarimetry and high-performance liquid-phase chiral separation methods. By virtue of solid-state analytical technologies including a powder X-ray diffraction (XRD), differential scanning calorimetry (DSC), thermal gravimetric analysis (TGA), a Karl Fischer titration and Fourier transform-attenuated total reflectance (FT-ATR) infrared spectroscopy, the crystalline forms of dihydromyricelin are characterized. The polymorph screening of dihydromyricelin is of important significance for the development of processes of active pharmaceutical ingredients, the development of dosage forms such as solid preparation, semi-solid preparation and suspension and quality control.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Gravimetric moisture measurement instrument
InactiveCN101101252AWeighing by removing componentSpecial purpose weighing apparatusMeasuring instrumentMoisture measurement
A measuring instrument for gravimetric moisture determination of a sample has a radiator, a weighing cell, and a sample receiver which can be connected to the weighing cell. The sample receiver allows the sample to be placed on or removed from the sample receiver. The radiator is positioned either above or below the sample, or both above and below, relative to the load direction of the weighing cell. A rotatably mounted heat conductor whose axis of rotation is orthogonal to the plane in which the sample or the sample receiver extends is positioned between the radiator and the sample. The heat conductor absorbs at least part of the radiation that impinges upon it and releases the absorbed radiation to the sample through at least one radiation-releasing surface. Through its ability to rotate, the heat conductor irradiates the entire sample surface.
Owner:METTLER TOLEDO INC
Quality detection methods of pearl powder and application thereof
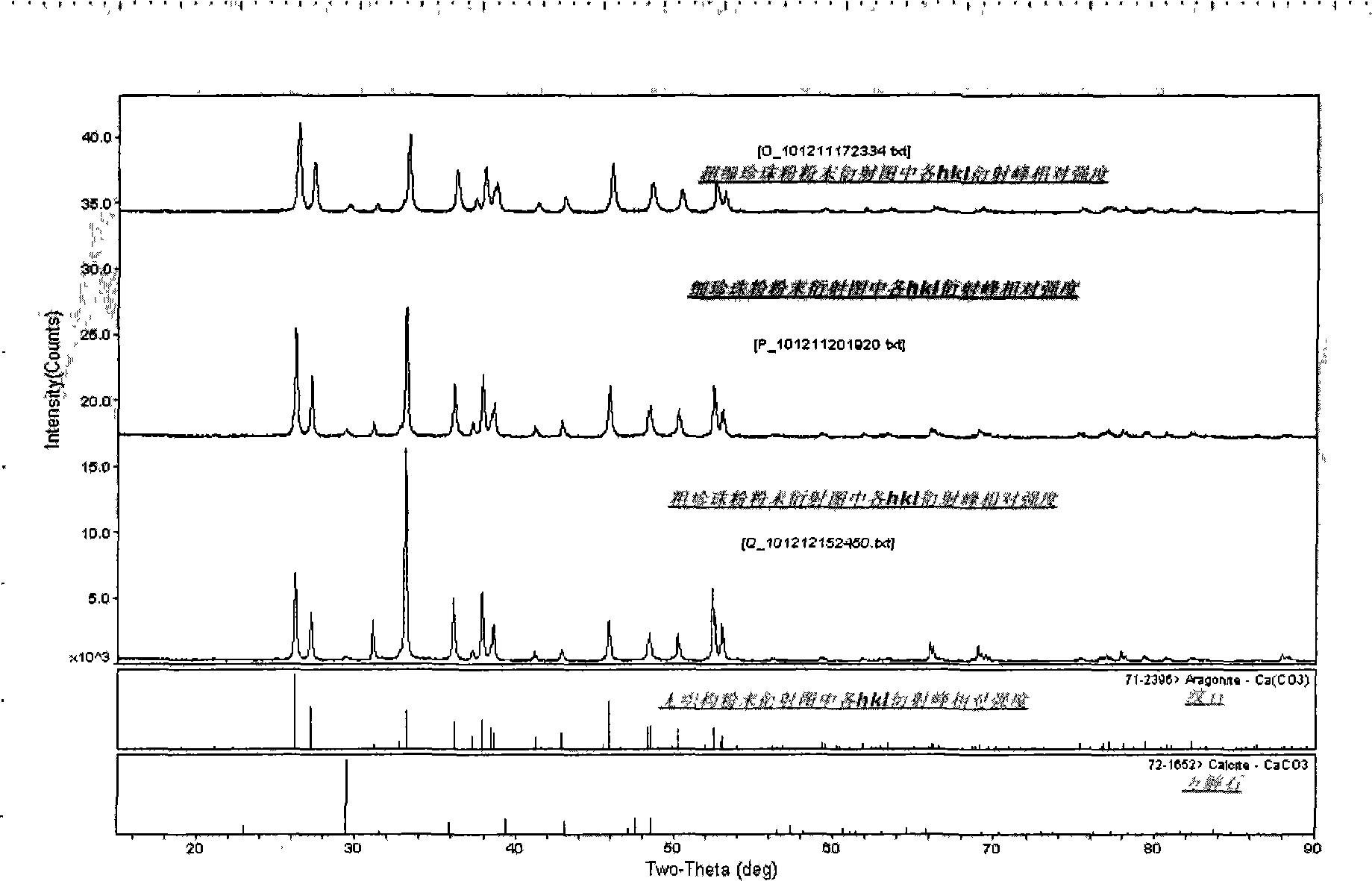
InactiveCN102323280AMaterial weighingMaterial analysis using radiation diffractionX-rayCurative effect
The invention relates to a plurality of quality detection methods of pearl powder and application thereof. The quality detection methods of the pearl powder comprise any one or the combination of the following methods: 1, analyzing the pearl powder by an XRD (X-Ray Diffraction) diffraction method, wherein the content of a calcite phase in the pearl powder is less than or equal to 4.0 weight percent and the content of an aragonite phase in the pearl powder is more than or equal to 96.0 weight percent; and / or, 2, analyzing the pearl powder by DSC (Differential Scanning Calorimetry) / TGA (Thermal Gravimetric Analysis), wherein the content of organic matter in the pearl powder is in the range of 3.5 to 5.0 percent; and / or, 3, after heating the pearl powder to a temperature of 400 DEG C plus and minus 5 DEG C, analyzing the pearl powder by the XRD diffraction method so as to detect that in the pearl powder, the percentage content of the aragonite phase which is changed into calcite is in the range of 10.0 percent to 15.0 percent. By the quality detection methods provided by the invention, the pearl powder and the quality thereof can be identified, the pearl powder can be obviously distinguished from nacre powder and mixed powder, so that the controllability of the quality of the pearl powder is improved and the curative effect and the safety of the pearl powder are ensured.
Owner:浙江长生鸟健康科技股份有限公司
Dispersion comprising metallic, metal oxide or metal precursor nanoparticles
ActiveUS9275773B2Stable and concentrated dispersionMetal-working apparatusInksChemical LinkageNanoparticle
The present invention relates to a dispersion comprising metallic, metal oxide or metal precursor nanoparticles and a polymeric dispersant, the dispersant comprising an anchor group with affinity for the metallic, metal oxide or metal precursor nanoparticles that is chemically bonded to a polymeric backbone characterized in that the dispersant has a 95 wt. % decomposition at a temperature below 300° C. as measured by Thermal Gravimetric Analysis. It further relates to metallic fluids or inks prepared from the dispersion and to the preparation of the dispersion and the metallic fluid or inks.
Owner:AGFA GEVAERT AG
Stable apremilast crystalline form ii free of solvate and method of making the same
ActiveUS20170298018A1Easy to prepareIncreased formationSenses disorderNervous disorderPhysical chemistryPharmaceutical drug
A stable Crystalline Form II of non-solvate of Apremilast (Formula I), methods of making Form II, pharmaceutical compositions comprising Form II, and their uses are disclosed. Also discloses are mixed crystals comprising Form Hand Form B and methods of making the same. The crystalline forms are characterized using X-ray powder diffractometry (XRPD), infrared spectroscopy (IR), differential scanning calorimetry (DSC), and thermal gravimetric analysis (TG). As compared with Forms A, B, C, D, E, F, and G reported in prior art references, Apremilast Form II of the present invention is more stable to temperature, light, and humidity, and is more suitable for long term storage; the crystallization solvents are safe and can be easily removed; the Form II has a white or off white appearance, and can be directly used in preparation processing; the preparation methods are simple and easy to reproduce, and are suitable for industrialized production.
Owner:UTOPHARM SHANGHAI +1
Thermosetting foam with improved thermal insulation and flame retardancy, and preparation method therefor
InactiveCN104540885ALow thermal conductivityHigh compressive strengthPolymer scienceThermal insulation
Provided is a thermosetting foam comprising: a polyisocyanurate foam formed by polymerizing a polyol-based compound and an isocyanate-based compound; a nucleating agent; and a flame retardant, wherein the thermal decomposition initiation temperature of the thermosetting foam measured by thermogravimetric analysis is 310 ℃ or higher. In addition, provided is a method for preparing a thermosetting foam having the thermal decomposition initiation temperature measured by thermogravimetric analysis of 310 ℃ or higher, comprising the steps of: mixing a polyol-based compound and a nucleating agent; adding a flame retardant to the mixture of the polyol-based compound and the nucleating agent; stirring an isocyanate-based compound with the mixture obtained by adding the flame retardant thereto to polymerize a polyisocyanurate.
Owner:LG HAUSYS LTD
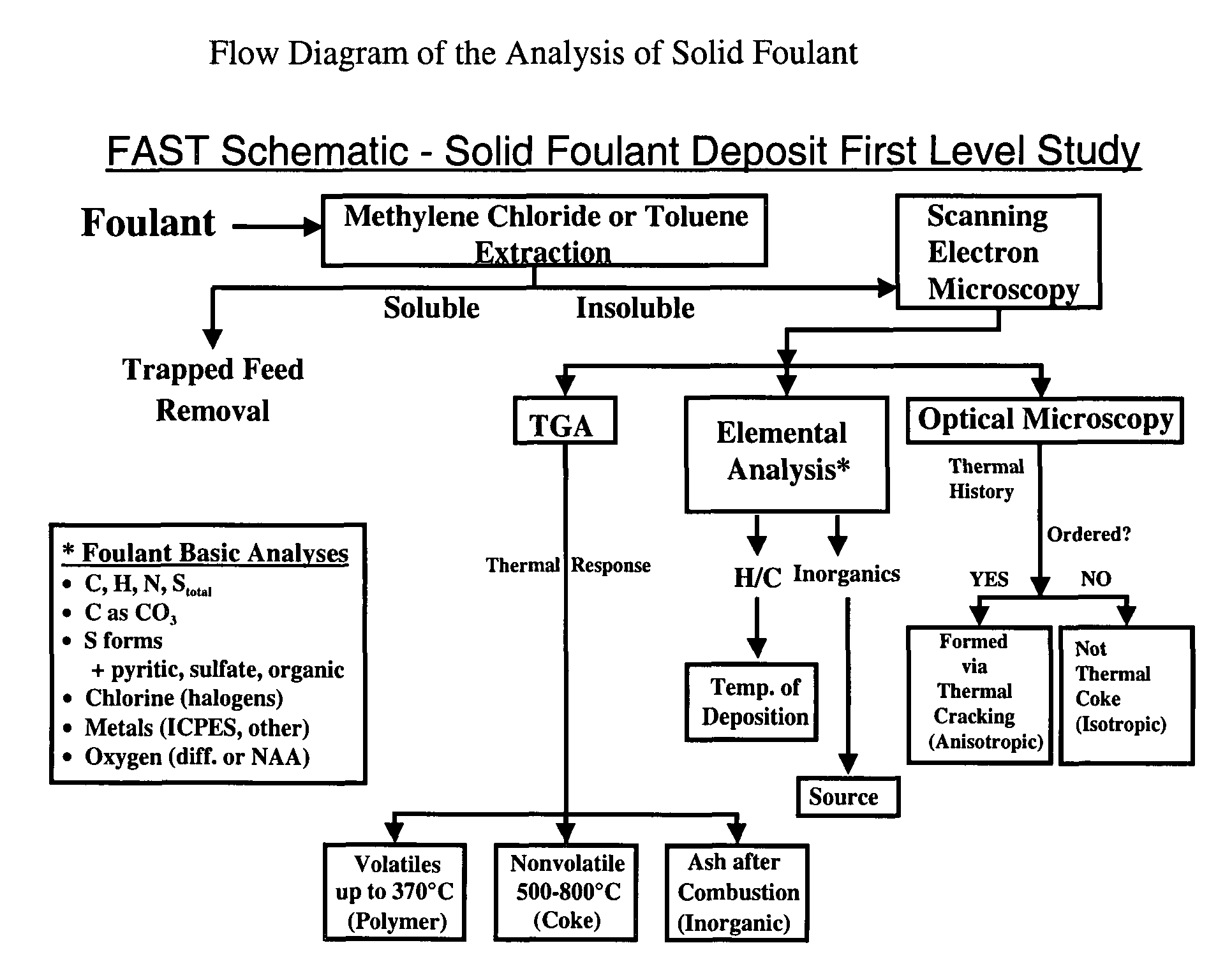
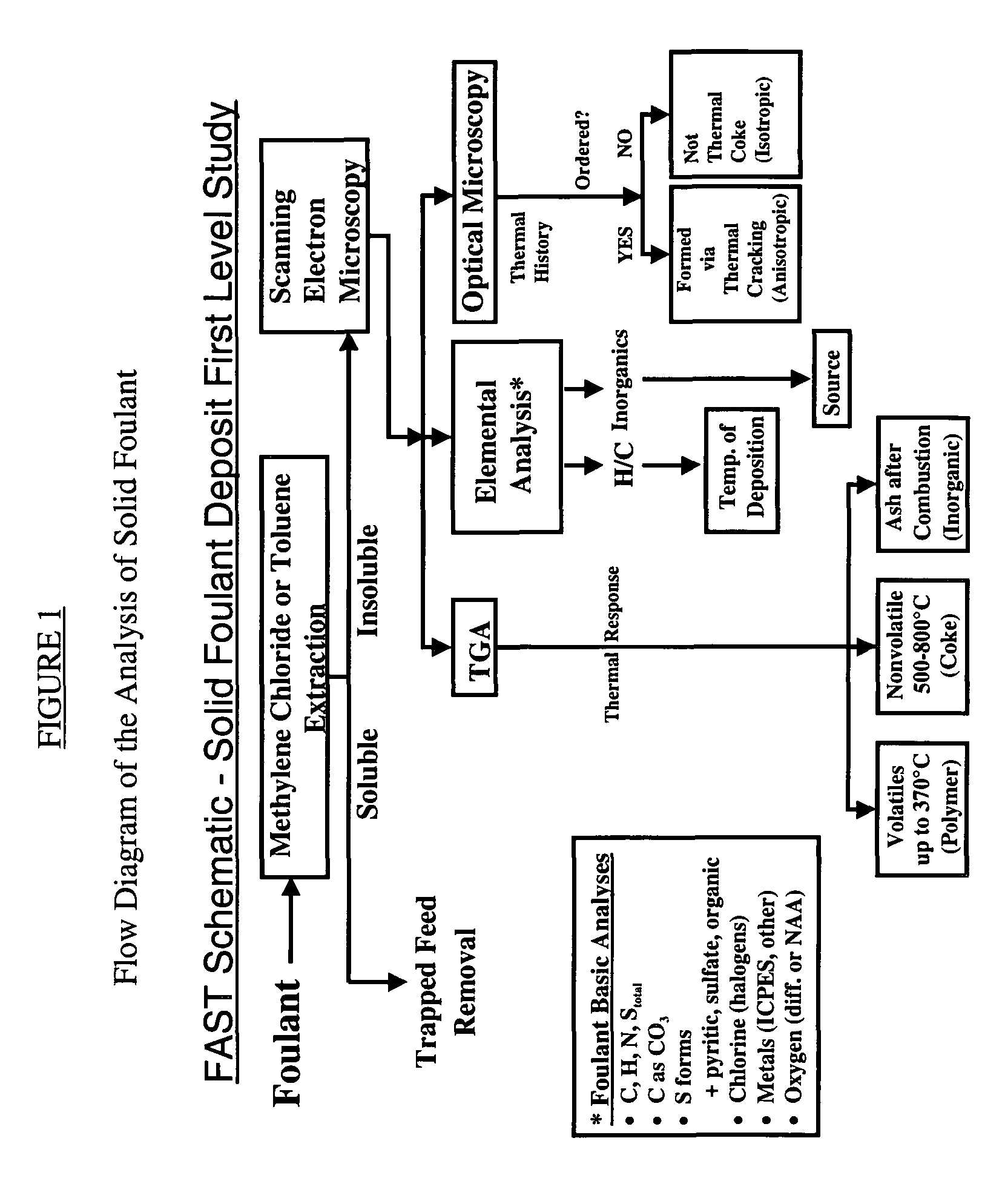
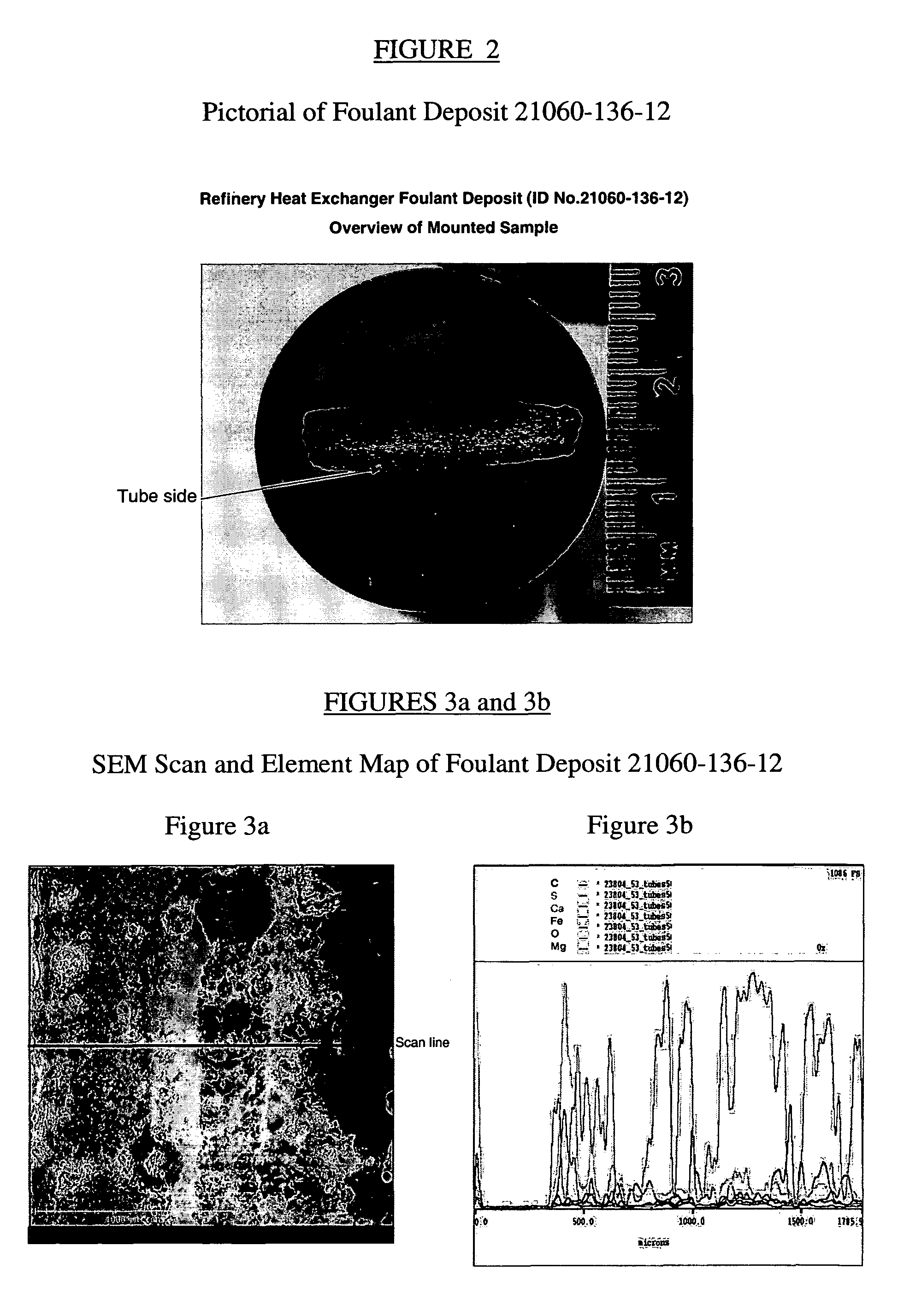
Method for refinery foulant deposit characterization
The present invention is a method to identify a refinery solid foulant of unknown composition including the following steps: obtaining a solid foulant sample, removing trapped feed from the sample with a solvent to obtain an insoluble sample, scanning the insoluble sample with a scanning electron microscope and energy dispersive x-rays, performing a thermal gravimetric analysis including an ash test on the insoluble sample to determine the presence of polymer, coke and inorganic elements, performing an elemental analysis on the insoluble sample for the elements carbon, hydrogen, sulfur, nitrogen, halogens, and metals, performing an optical microscopy on the insoluble sample to determine the presence of wax, asphaltenes, anisotropic coke and isotropic coke, and identifying the solid foulant.
Owner:EXXON RES & ENG CO
Vanadium battery electrode treatment method
InactiveCN103199271ALower resistanceLarge exchange currentCell electrodesState of artElectrical resistance and conductance
The invention provides a vanadium battery electrode treatment method. The method comprises the following steps of: (1) under inert atmosphere, carrying out graphitizing treatment on carbon felt at a preset temperature in preset duration to obtain graphite felt; (2) finding an appropriate heat treatment temperature range using differential thermal analysis and thermal gravimetric analysis, and then carrying out heat treatment on the graphite felt; (3) testing the electrode of the heat treated graphite felt to obtain an optimum condition; and (4) in the optimum condition, carrying out heat treatment on the graphite felt to obtain the vanadium battery electrode. Compared with the prior art, the vanadium battery electrode obtained by the vanadium battery electrode treatment method has a relatively small resistance and a relatively large exchange current, and relatively good electric properties.
Owner:朴宁
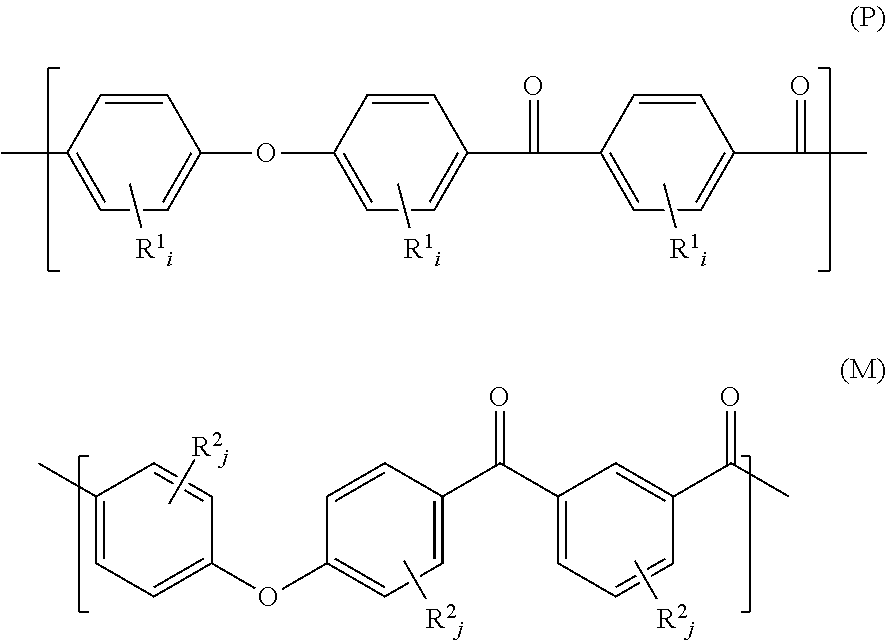
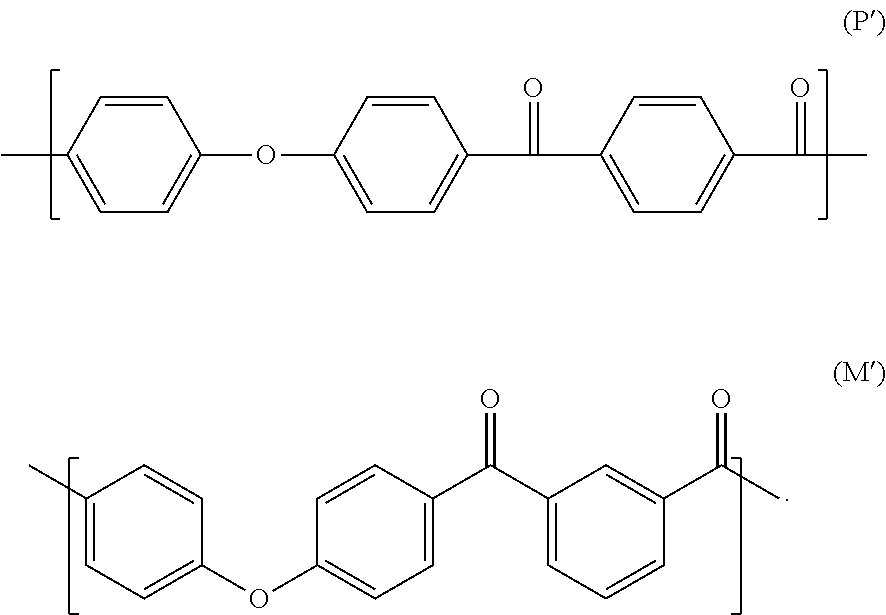
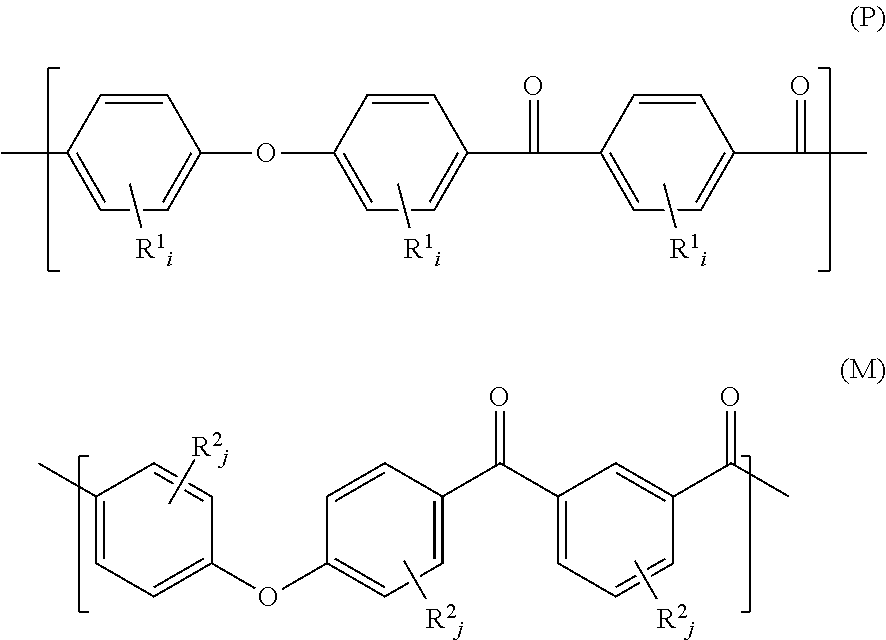
Block Copolymer Composition
A block copolymer composition is disclosed herein. In some embodiments, a block copolymer composition includes a diblock copolymer and a triblock copolymer, each including a polyolefin-based block and a polystyrene-based block, wherein the polyolefin-based blocks are present between 45 wt % to 90 wt %, wherein the polystyrene-based blocks are present between 10 wt % to 55 wt %, wherein the difference (ΔT) between a thermal decomposition initiation temperature and a thermal decomposition termination temperature (ΔT) measured by Thermo-Gravimetric Analysis (TGA) is 55° C. or greater, and the diblock copolymer and the triblock copolymer do not have a residual unsaturated bond.
Owner:LG CHEM LTD
Crystal form of salt formed from vinpocetine and phosphoric acid, and preparation method
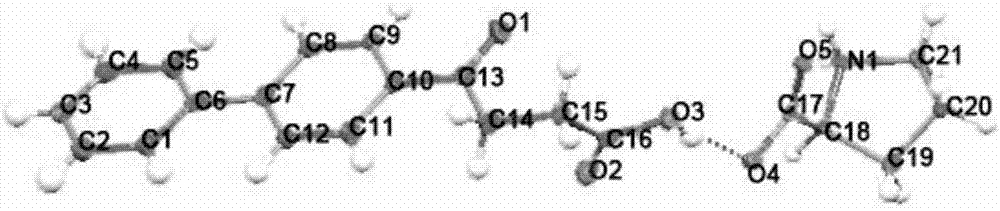
InactiveCN105153147AImprove solubilityImprove stabilityOrganic chemistry methodsSolubilityO-Phosphoric Acid
The invention discloses a crystal form of a salt formed from vinpocetine and phosphoric acid, and a preparation method, and relates to the technical field of discovery and preparation of a crystal form of a pharmaceutical salt. The prepared salt formed from vinpocetine and phosphoric acid is detected through analytical methods, such as thermal gravimetric analysis (TGA), X-ray powder diffraction (P-XRD), X-ray single crystal diffraction (S-XRD), differential scanning calorimetry (DSC), and infrared spectroscopy (IR). Vinpocetine is a indissolvable medicine (the solubility of which is about 5 [mu]g / mL and pKa of which is 7.31), while it is proved through detection that the salt prepared from vinpocetine and phosphoric acid has excellent solubility and stability. Development of the novel salt form establishes foundation for development and application of new formulation.
Owner:JIANGSU QINGJIANG PHARMA
Stable apremilast crystalline form II free of solvate and method of making the same
ActiveUS9850205B2Increased formationPromote formationSenses disorderNervous disorderPhysical chemistryPharmaceutical drug
A stable Crystalline Form II of non-solvate of Apremilast (Formula I), methods of making Form II, pharmaceutical compositions comprising Form II, and their uses are disclosed. Also discloses are mixed crystals comprising Form Hand Form B and methods of making the same. The crystalline forms are characterized using X-ray powder diffractometry (XRPD), infrared spectroscopy (IR), differential scanning calorimetry (DSC), and thermal gravimetric analysis (TG). As compared with Forms A, B, C, D, E, F, and G reported in prior art references, Apremilast Form II of the present invention is more stable to temperature, light, and humidity, and is more suitable for long term storage; the crystallization solvents are safe and can be easily removed; the Form II has a white or off white appearance, and can be directly used in preparation processing; the preparation methods are simple and easy to reproduce, and are suitable for industrialized production.
Owner:UTOPHARM SHANGHAI +1
Method for measuring disintegration of fibrous product
The present invention provides a method and system for measuring disintegration of a fibrous product. The method comprises disintegration of a sample of the fibrous product in an aqueous solution, optionally promoted by mechanical energy, passing the aqueous solution through a screen to obtain a permeate and retained fraction on the screen, and analyzing at least one parameter from the permeate for example by a gravimetric analysis, an optical analysis, an electrochemical analysis, a volumetric analysis, or combination thereof. Advantages include adjustabilxity, speed, and high correlation with actual flushability or repulpability of the fibrous product, and utility in a process for manufacturing a fibrous sheet exhibiting con- trolled disintegration, such as a flushable or repulpable fibrous sheet.
Owner:KEMIRA OY
Preparation of 9,9-bis(6-hydroxynaphthalene-2-yl) fluorene crystal without complex residual solvent
ActiveCN108586205AEasy to implementQuality improvementOrganic compound preparationOrganic chemistry methodsPolyesterX-ray
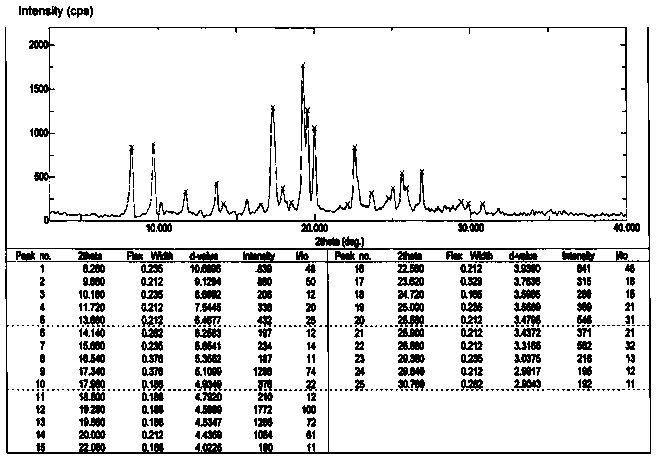
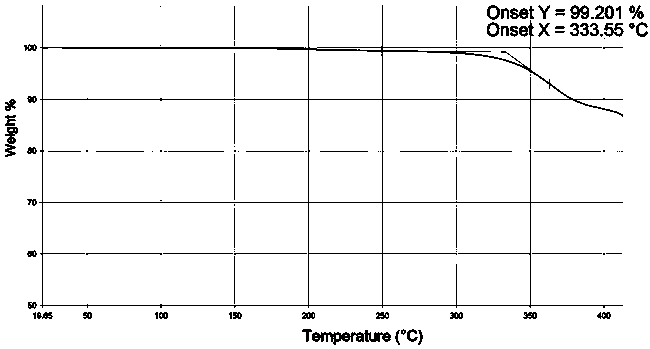
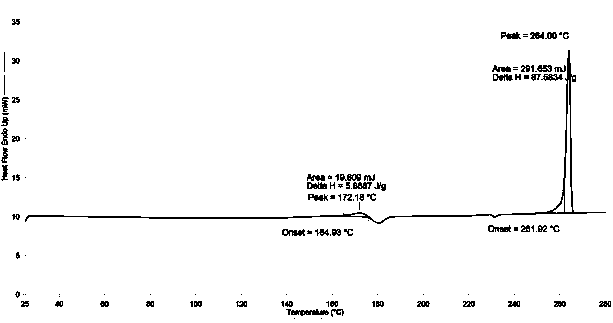
The invention discloses a 9,9-bis(6-hydroxynaphthalene-2-yl) fluorene crystal without complex residual solvent and is obtained through beating a low-boiling-point chlorine-containing solvent and carrying out vacuum drying treatment. The thermal gravimetric analysis (TGA) spectrum shows that no complex residual solvent exists, and the melting endothermic peak on the differential scanning calorimeter (DSC) spectrum is 260-266 DEG C. The characteristic 2-theta values of the X-ray powder diffraction (XRPD) analysis are as follows: 8.3+ / -0.20 (48), 9.7+ / -0.20 (50), 17.3+ / -0.20 (74), 19.3+ / -0.20 (100), 19.7+ / -0.20 (72), 20.0+ / -0.20 (61), 22.6+ / -0.20 (48) and 26.9+ / -0.20 (32). The crystal without complex residual solvent has the advantages that when materials of polycarbonate, polyester, polyimide and the like are prepared by adopting a solvent-free polycondensation process, the release and dissipation of residual toxic and harmful solvents are prevented, the air environment is prevented frombeing polluted, and the quality performance of polymer materials is not seriously influenced by the release of encapsulated solvent in the reaction.
Owner:JIANGSU EVER GALAXY CHEM CO LTD
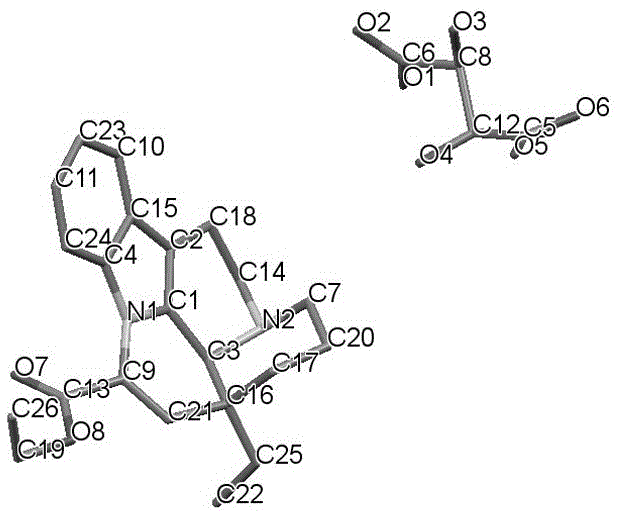
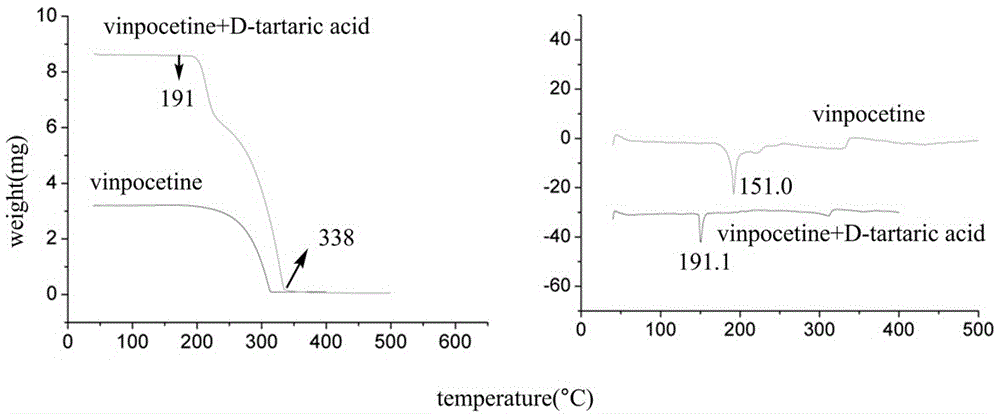
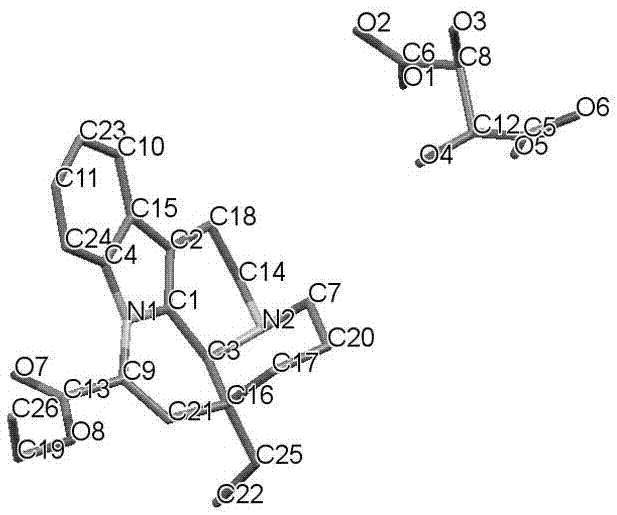
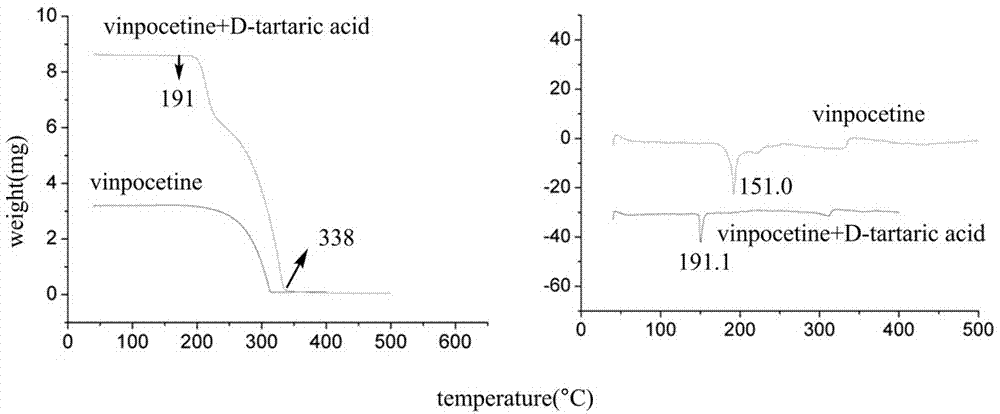
Crystal form of salt formed by vinpocetine and D-tartaric acid and preparation method of crystal form
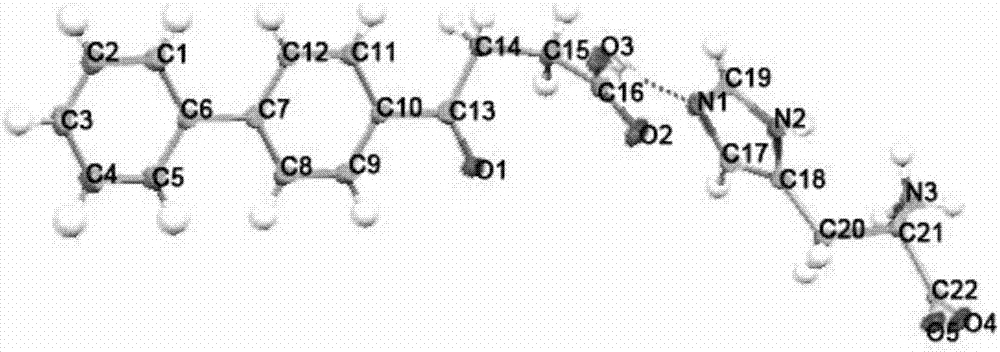
The invention relates to a crystal form of salt formed by vinpocetine and D-tartaric acid and a preparation method of the crystal form and belongs to the technical field of the discovering and preparation of the crystal form of medicine salt. The salt prepared by the method is detected as the salt formed by the vinpocetine and the D-tartaric acid through analyzing methods such as thermal gravimetric analysis (TGA), X-ray powder diffraction (P-XRD), X-ray single-crystal diffraction (S-XRD), differential scanning calorimetry (DSC) and infrared spectroscopy (IR). The vinpocetine is an insoluble drug (the solubility is about 5 microgram / mL and pKa=7.31), tests show that the salt formed by the vinpocetine and the D-tartaric acid is good in solubility and stability in the preparing process of solid preparations, and the development of new salt crystals lays a foundation for the development and application of the new dosage form of the salt. The spatial structure of the salt formed by the vinpocetine and D-tartaric acid is as shown in picture 1.
Owner:JIANGSU QINGJIANG PHARMA
Method used for detecting absorbing capacity of carbon dioxide absorbed from ethanolamine
ActiveCN106370776ASmall source restrictionsEasy to transportChemical analysis using titrationCo2 absorptionTurbidity
The invention relates to a method used for detecting absorbing capacity of carbon dioxide absorbed from ethanolamine. The carbon dioxide is absorbed through multilevel sodium hydroxide until the last level titrated with calcium chloride finds no turbidity or sedimentation or extremely little turbidity, then sedimentation is conducted through superfluous calcium chloride solution, and finally weighing and calculation on obtained CaCO3 are conducted. The absorbing capacity of the carbon dioxide absorbed from the ethanolamine can be accurately and stably detected through the multilevel detection titration. The method to detect the absorbing capacity of the carbon dioxide absorbed from the ethanolamine in a laboratory through the multilevel sodium hydroxide has an obvious measurement effect, gravimetric analysis has little error and experimental result is stable and accurate.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Electrode for non-aqueous electrolyte rechargeable battery, non-aqueous electrolyte rechargeable battery, and battery pack
ActiveCN103782416ANon-aqueous electrolyte accumulator electrodesLi-accumulatorsPeak areaFluorine containing
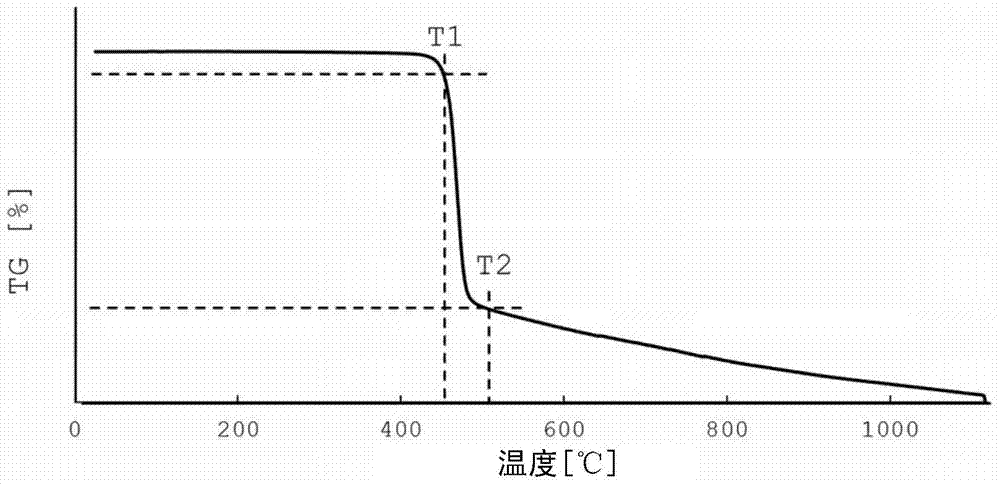
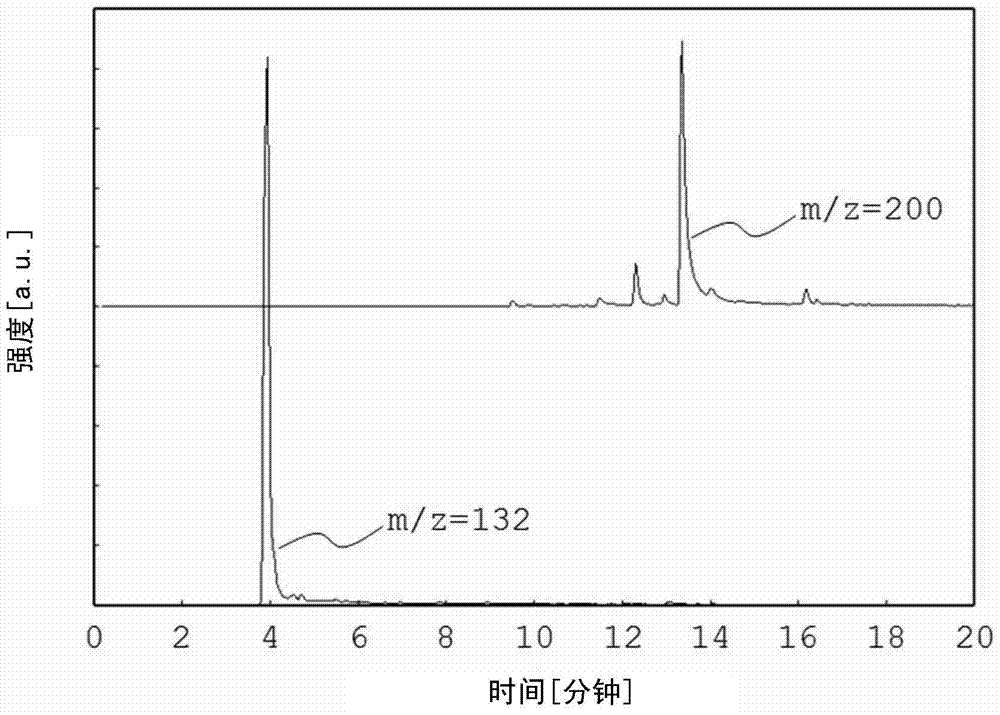
[Problem] To provide a non-aqueous electrolyte rechargeable battery having an excellent retention capacity. An electrode for a non-aqueous electrolyte rechargeable battery of an embodiment of the present invention: has an active material layer, which contains an active material and a fluorine-containing binder, and a current collector that is bound to the active material layer; and, in pyrolysis gas chromatography mass spectrometry at a pyrolysis temperature of (T1 + T2) / 2 DEG C (where T1 DEG C represents the temperature at which pyrolysis of the binding material starts, and T2 DEG C represents the temperature at which pyrolysis ends), a peak exists in an ion chromatogram at any of the mass numbers selected from at least 81, 100, 132 and 200, and, if the peak area at T1 DEG C is denoted as X, and the peak area at T2 DEG C is denoted as Y, X and Y satisfy the condition, 2X>= Y. When the binding material is analyzed by thermogravimetry, the temperature at which pyrolysis of the binding material starts is the temperature at which, in the main weight loss process, there is a 5% loss of weight during the weight loss process. When the binding material is analyzed by thermogravimetry, the temperature at which pyrolysis of the binding material ends is the temperature at which, in the main weight loss process, there is a 95% loss of weight during the weight loss process. The peak area is the area of the peak at the mass number that provides the maximum area among ion chromatographs extracted at mass numbers 81, 100, 132 and 200 in pyrolysis gas chromatography mass spectrometry at a pyrolysis temperature of the binding material alone of (T1 + T2) / 2 DEG C.
Owner:KK TOSHIBA
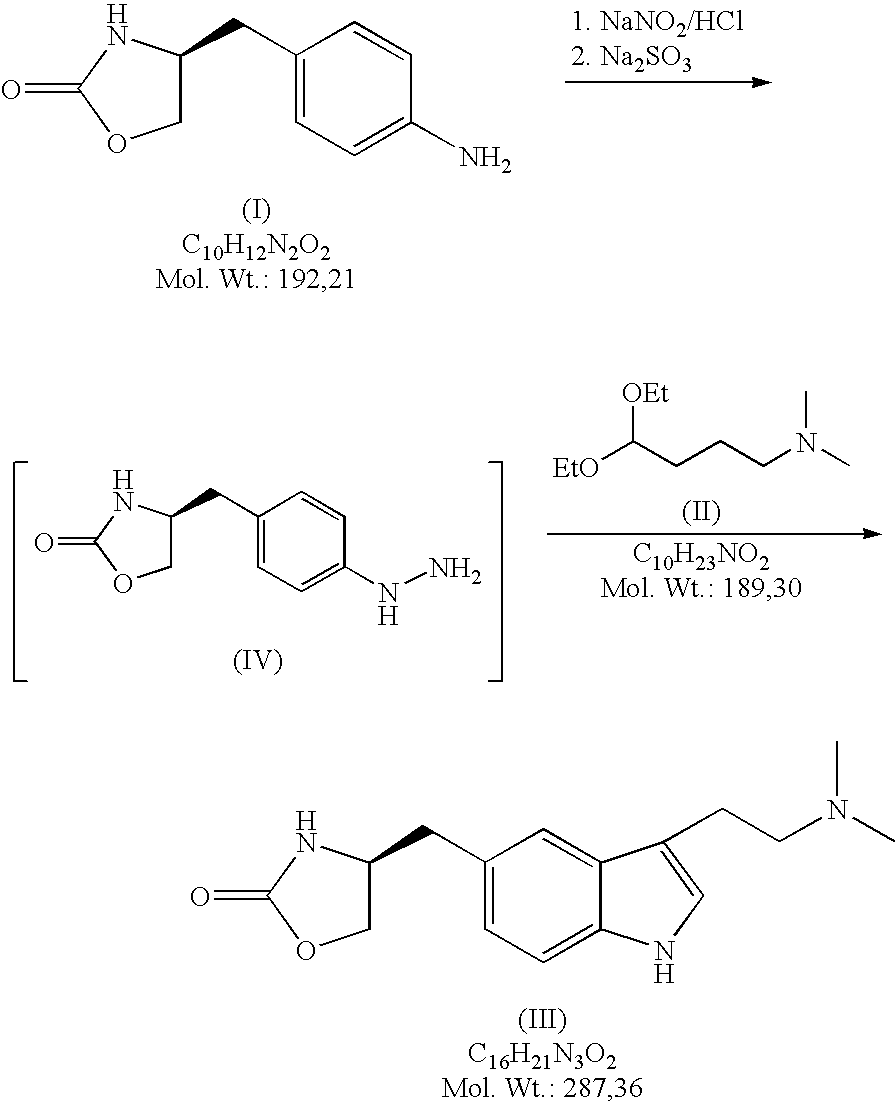
Method for the preparation of zolmitriptan
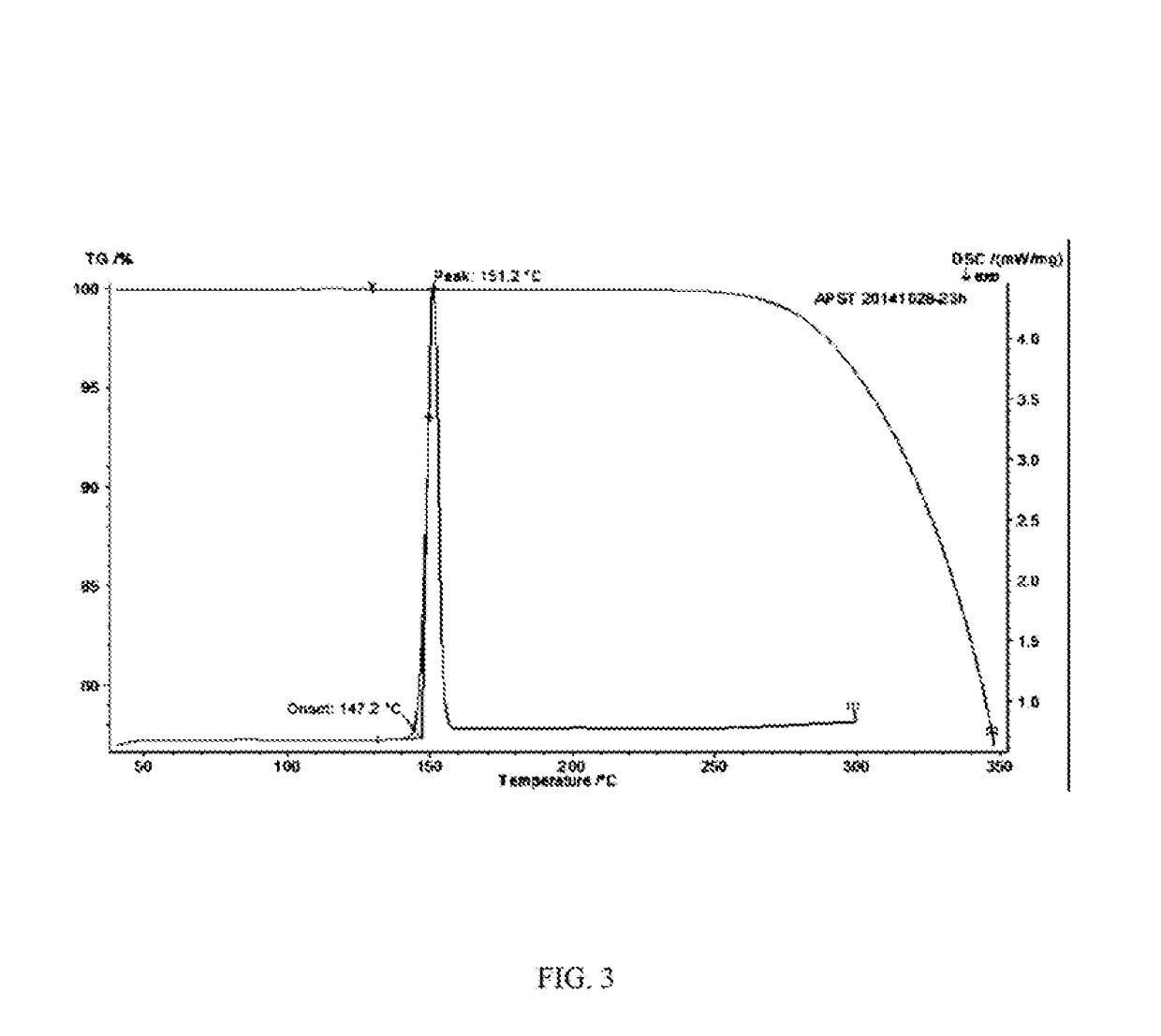
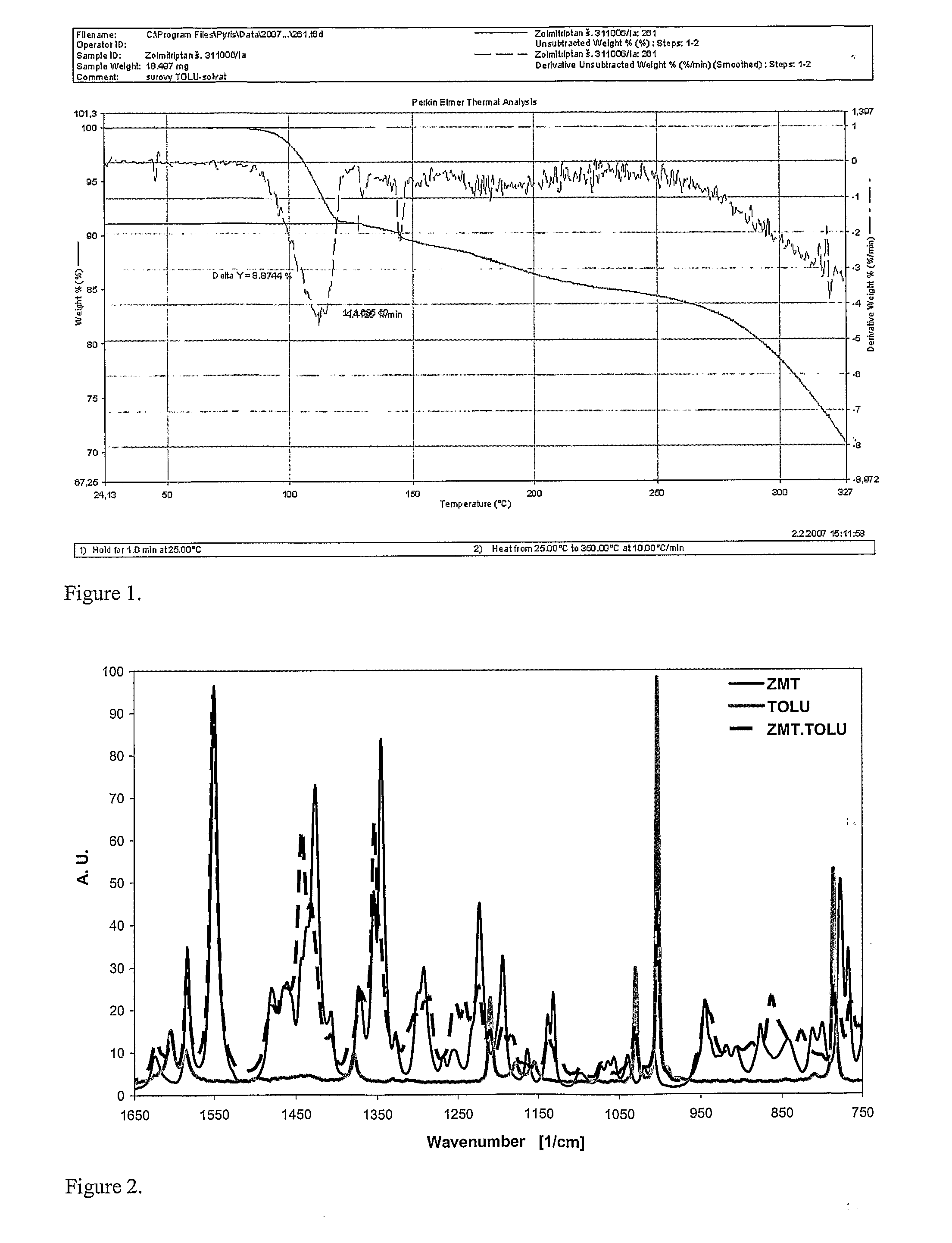
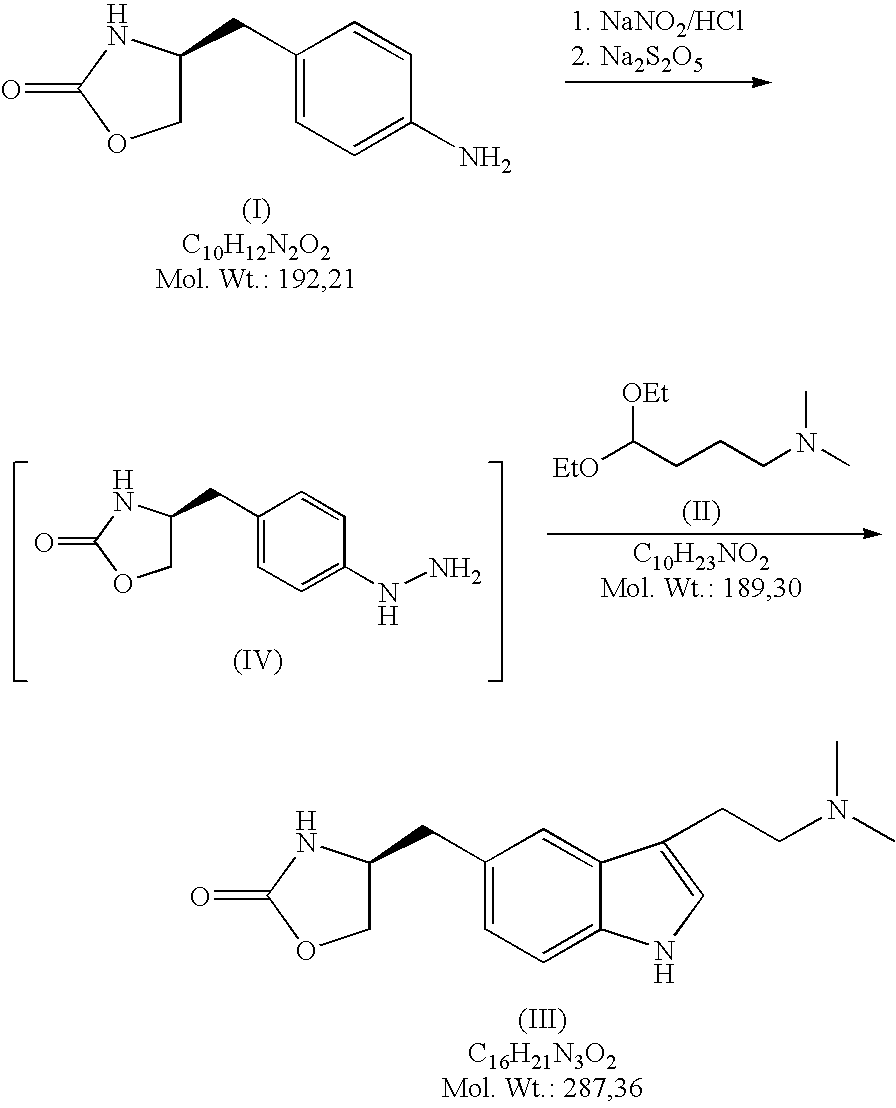
In the preparation of zolmitriptan of formula III the reduction of the diazonium salt to (5)-4-(4-hydrazinobenzyl)-1,3-oxazolidin-2-one of formula IV is performed in a more concentrated mixture and by the effect on an alkali metal disulphite, preferably sodium disulphite. A zolmitriptan toluene solvate, characterized by a toluene content of 9 to 14% by weight according to the gas chromatography determination and by a maximum of the corresponding mass loss at temperatures of about 111° C. in the gravimetric analysis record. A zolmitriptan toluene solvate, showing strong Raman bands at the wave numbers of 1443 and 1354 cm−1, characteristic for the crystal lattice of zolmitriptan with built-in toluene, and further marked bands at 1004 and 786 cm−1, characteristic for toluene.
Owner:ZENTIVA AS
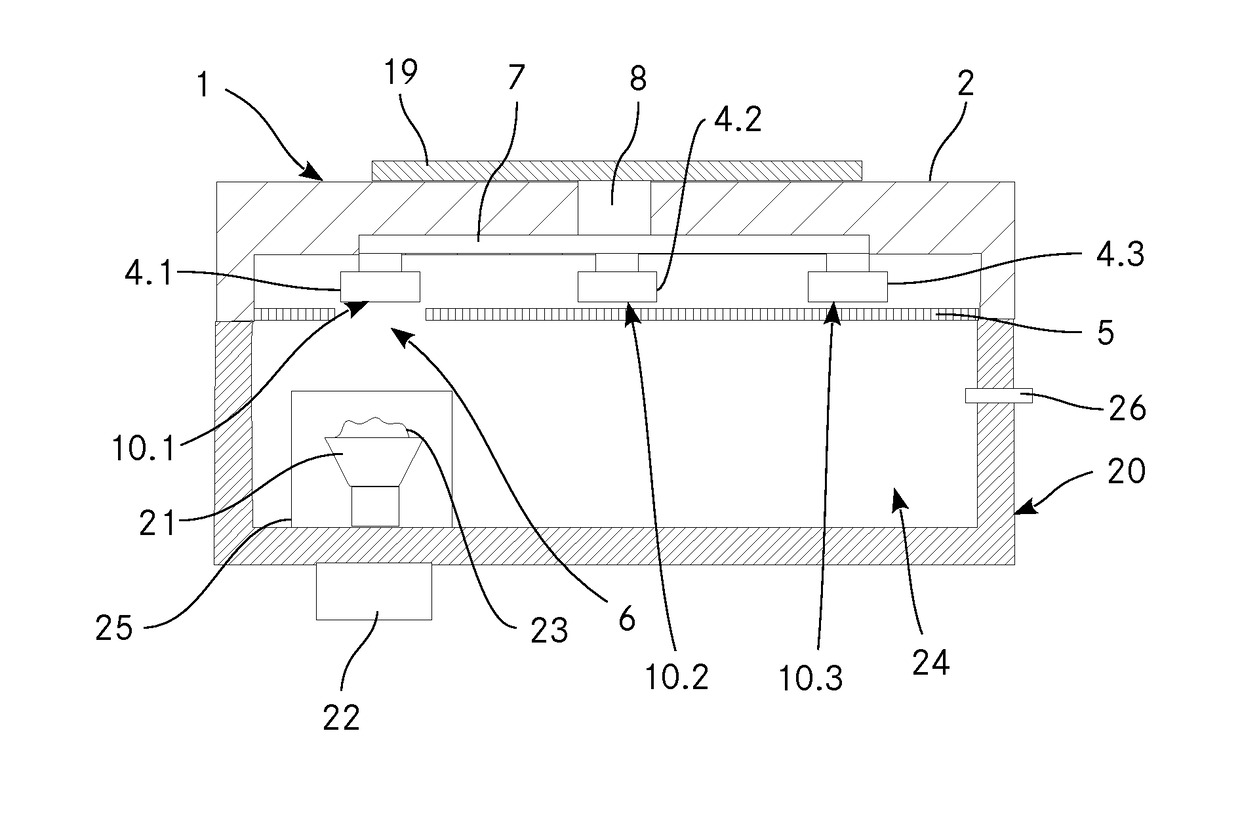
Coupling device for thermogravimetric analysis
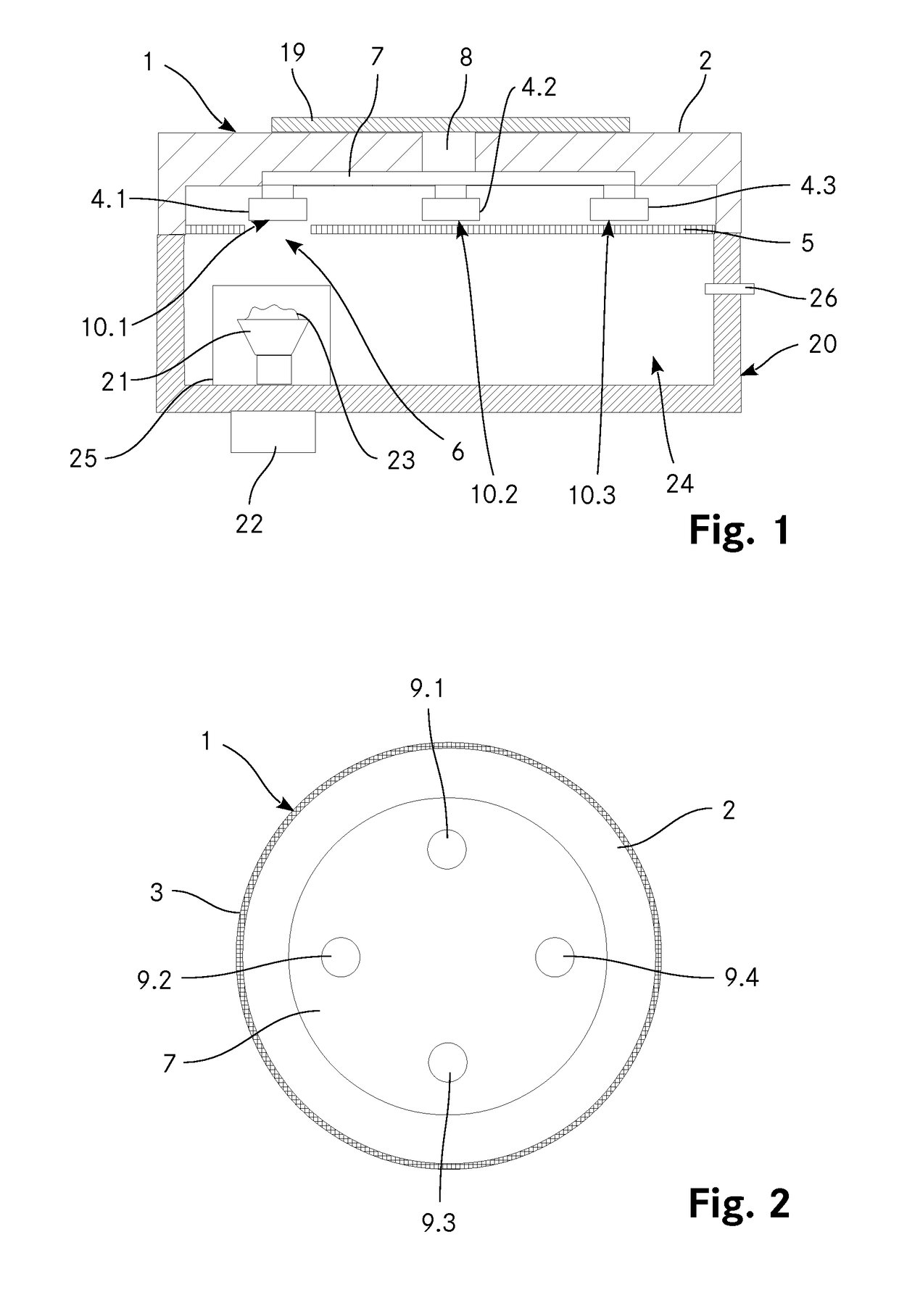
InactiveUS20180149569A1Thermometer detailsWeighing by removing componentEngineeringThermogravimetric analysis
The invention relates to a coupling device for thermogravimetric analysis for coupling a thermogravimetric analysis with a spectroscopic analysis, comprising a housing with a connecting element by means of which said housing can be connected in a gas-tight and detachable manner to a sample chamber of a device for gravimetric analysis. The coupling device comprises at least two flange bushings or at least two adsorption elements which are detachably connected to the coupling device and comprise, on a first side, a condensation surface or an adsorption body for gaseous components. A screen is arranged on the coupling device such that it lies between the at least two flange bushings or the at least two adsorption elements and the sample chamber, wherein the screen has at least one opening. A changing device allows the at least two flange bushings, the at least two adsorption elements or the screen to move in such a way that the condensation surface of at least one flange bushing or an adsorption body of at least one adsorption element lies opposite the at least one opening. A cooling device allows the condensation surfaces of the at least two flange bushings to be cooled.
Owner:GLAS TROSCH HLDG
Fenbufen eutectic and preparation method and application thereof
ActiveCN107188799AEasy to carry outImprove solubilityOrganic chemistry methodsCarboxylic compound separation/purificationSolubilityX-ray
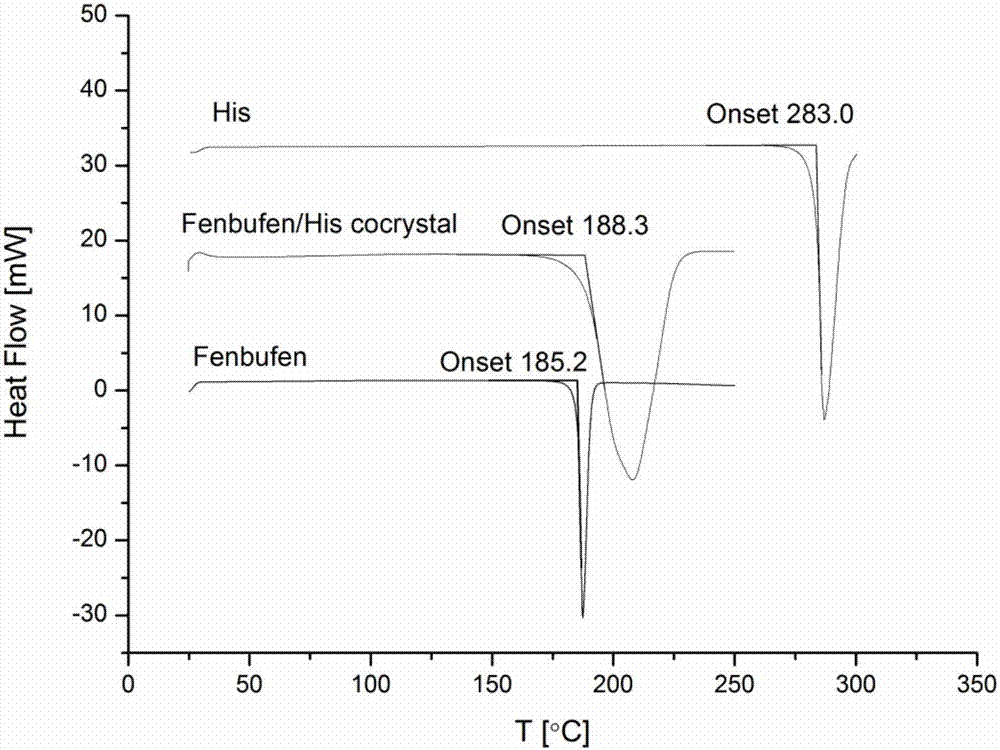
The invention provides fenbufen eutectic that is formed by bonding the pharmaceutical active ingredient fenbufen to an amino acid eutectic reagent by means of intermolecular hydrogen bonding; the amino acid eutectic reagent is L-histidine or L-proline. The invention also discloses a preparation method of the fenbufen eutectic; the preparation method comprises: using fenbufen and eutectic reagents as raw materials, and subjecting the raw materials to solvent-assisted grinding or suspending. 'Gold standard'-monocrystal X-ray diffraction determination of eutectic inspection for the fenbufen eutectic proves that the fenbufen eutectic is formed by connecting via noncovalent hydrogen bonds and is not salt but pharmaceutical eutectic; all acquired pharmaceutical eutectics are subjected to structural characterization by TGA (thermal gravimetric analysis), DSC (differential scanning calorimetry), infrared spectrometry, powder X-ray diffraction and other means, and the results prove that a novel form of eutectic is formed. Through the fenbufen eutectic, the problem that raw fenbufen has poor water solubility is solved; the fenbufen eutectic has better water solubility; the development of pharmaceutical clinical research of fenbufen is facilitated.
Owner:NAT INST FOR FOOD & DRUG CONTROL
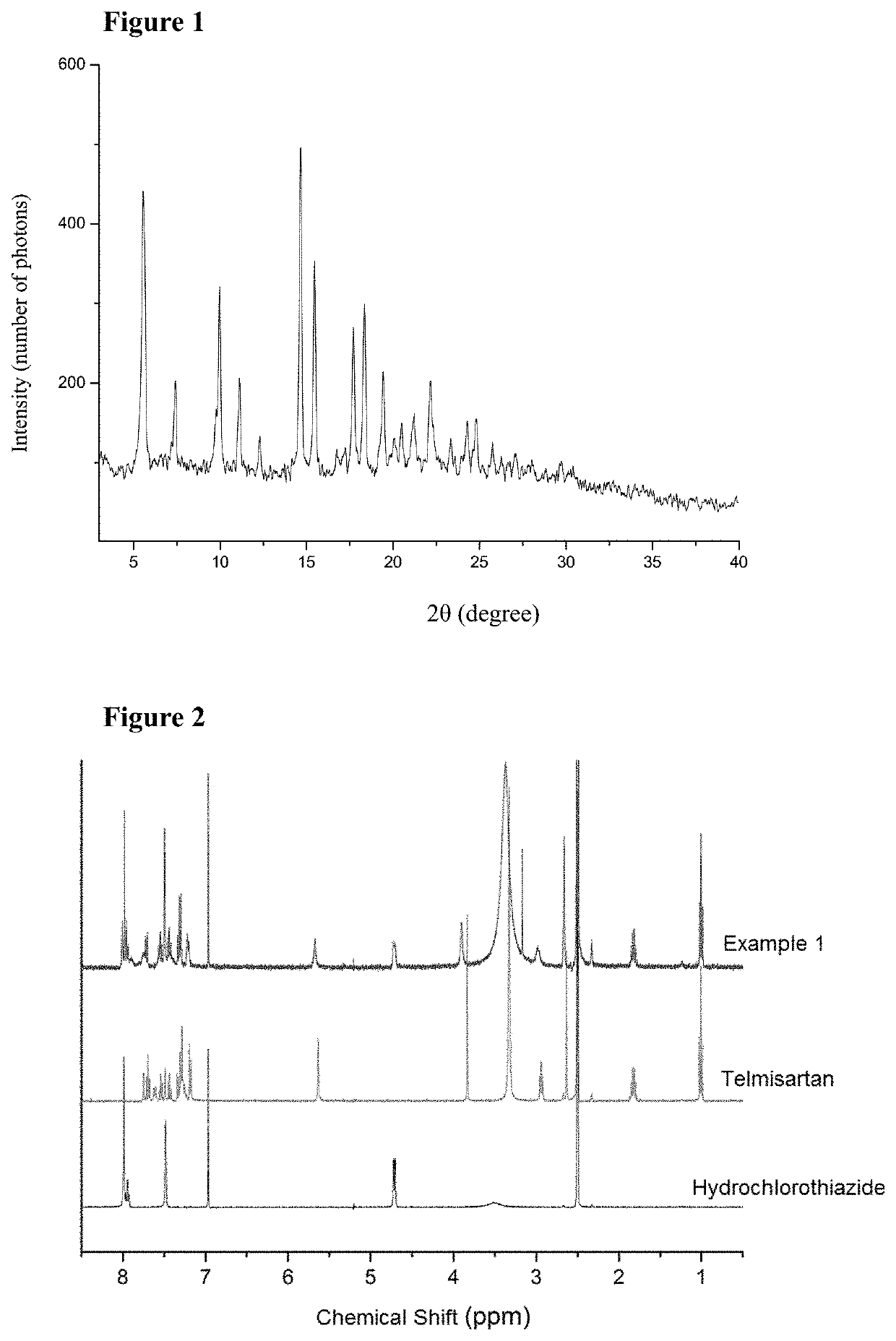
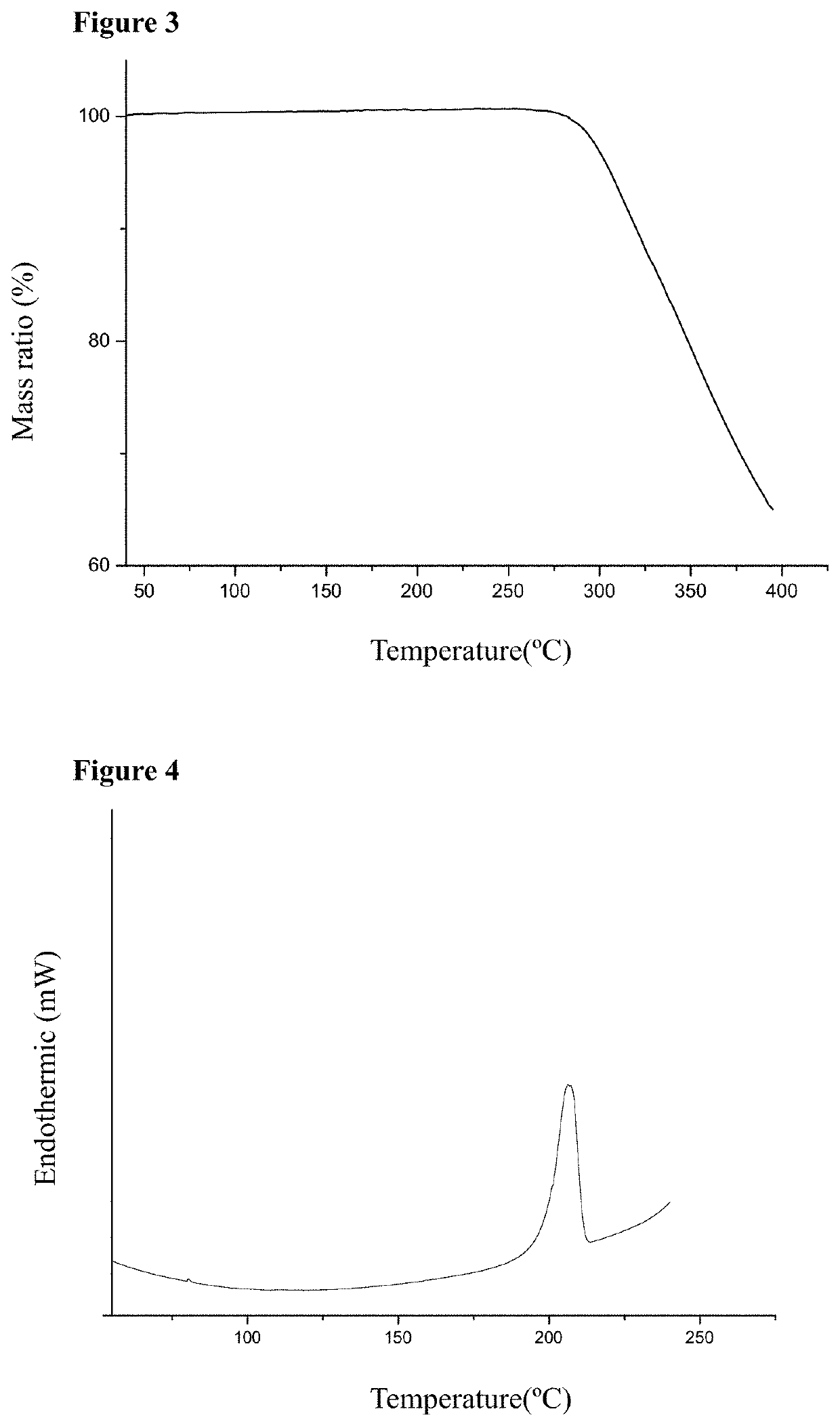
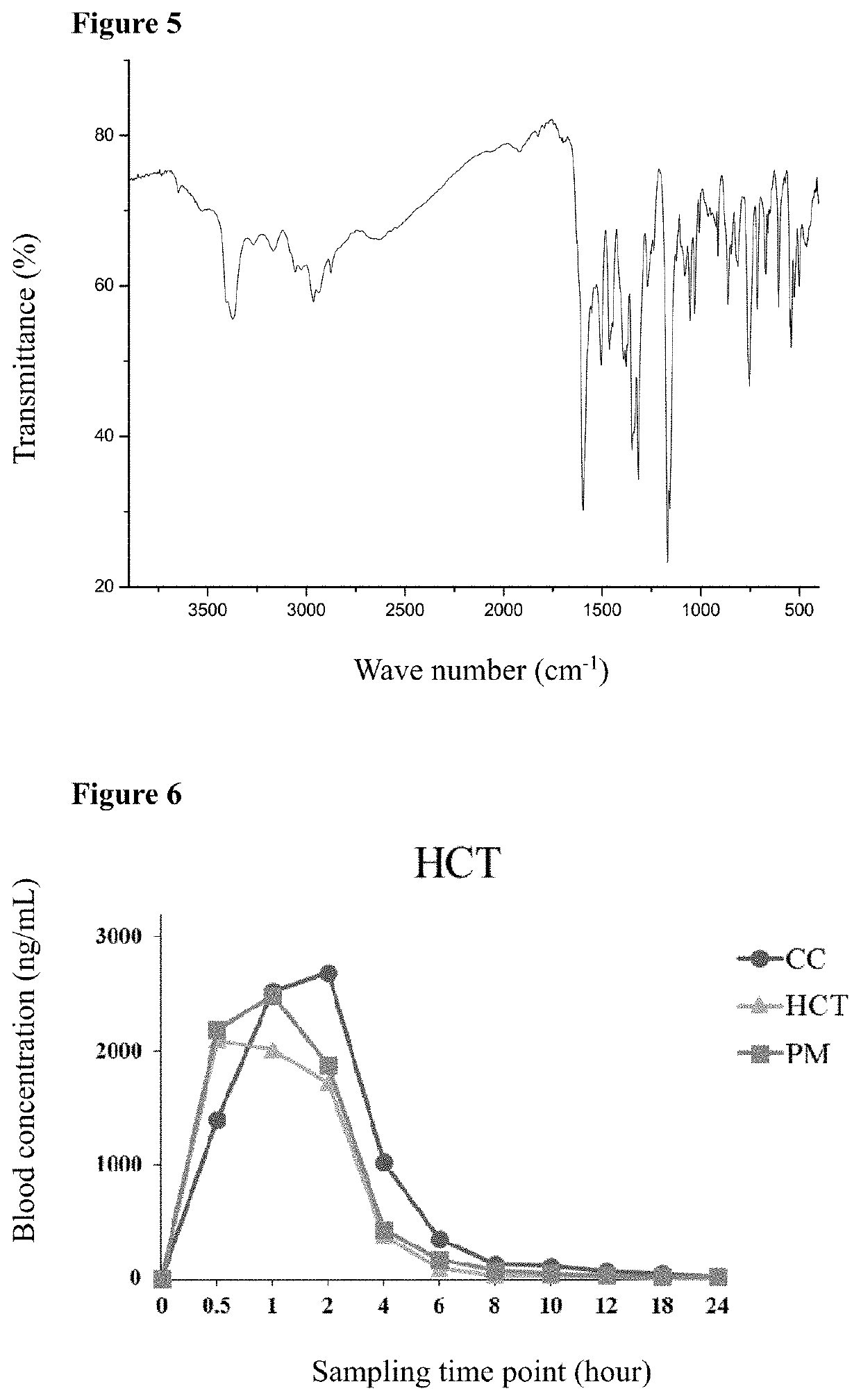
Cocrystal of Telmisartan and Hydrochlorothiazide
InactiveUS20200181121A1Easy to controlIncrease contentOrganic active ingredientsOrganic chemistry methodsFt ir spectraBlood plasma
The present disclosure relates to a cocrystal of telmisartan and hydrochlorothiazide, a preparation method and use thereof. In the cocrystal, the molar ratio of telmisartan and hydrochlorothiazide is 1:1. The cocrystal of telmisartan and hydrochlorothiazide was characterized by X-ray powder diffraction (XRPD), proton nuclear magnetic resonance spectra (1H-NMR), thermal gravimetric analysis (TG), scanning differential calorimetry (DSC) and infrared (IR) spectra, and it was found that the maximum plasma concentration of the cocrystal in SD rats was higher than that of any one of hydrochlorothiazide and telmisartan itself. The cocrystal of telmisartan and hydrochlorothiazide has a simple preparation method and good physical and chemical properties.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Process for producing polyether polyol
A process for producing at a high efficiency with high selectivity a polyether polyol reduced in coloration through the dehydrating condensation of a polyol. In producing a polyether polyol through the dehydrating condensation of a polyol, a solid acid catalyst satisfying at least one of the following (1) to (3) is used: (1) the acidity function H0 as measured by Hammett's indicator adsorption method is larger than -3; (2) in analysis by temperature programmed ammonia desorption (TPD), the amount of ammonia desorbed in the region of 100-350 DEG C is at least 60% of the amount of ammonia desorbed in the whole test region (25-700 DEG C region); and (3) in a thermogravimetric (TG) analysis, the amount of water desorbed in the region of 32-250 DEG C is 3 wt.% or larger based on the basis weight.
Owner:MITSUBISHI CHEM CORP
Method for determining water-soluble chloride in feed
PendingCN114235628ASimplify the analysis processImprove accuracyWeighing by removing componentPhysical chemistryWater soluble
The invention relates to a method for determining water-soluble chlorides in feed, which comprises the following steps: 1) crushing a feed sample, adding the crushed feed sample into water, stirring, and fixing the volume with water to obtain a dispersion liquid; 2) filtering the dispersion liquid, transferring part of filtrate, adding a nitric acid solution and an excessive silver nitrate solution, and then separating to obtain a precipitate; and 3) cleaning the precipitate, drying in a dark place, cooling to room temperature in a dark place, weighing, and calculating to obtain the content of chloride in the feed sample. Compared with the prior art, the method adopts a gravimetric analysis method and is provided with an ion precipitation detection process, the determination method is simpler and more visual, the accuracy is higher, the repeatability is better, the operation is simpler, the reagent cost and the reagent safety risk are reduced, and the method can be used for detecting whether the chloride content of a product reaches the standard or not.
Owner:FUJIAN AONONG BIOLOGICAL TECH GRP CO LTD +2
Crystal form and preparation method of a salt formed by vinpocetine and d-tartaric acid
The invention relates to a crystal form of salt formed by vinpocetine and D-tartaric acid and a preparation method of the crystal form and belongs to the technical field of the discovering and preparation of the crystal form of medicine salt. The salt prepared by the method is detected as the salt formed by the vinpocetine and the D-tartaric acid through analyzing methods such as thermal gravimetric analysis (TGA), X-ray powder diffraction (P-XRD), X-ray single-crystal diffraction (S-XRD), differential scanning calorimetry (DSC) and infrared spectroscopy (IR). The vinpocetine is an insoluble drug (the solubility is about 5 microgram / mL and pKa=7.31), tests show that the salt formed by the vinpocetine and the D-tartaric acid is good in solubility and stability in the preparing process of solid preparations, and the development of new salt crystals lays a foundation for the development and application of the new dosage form of the salt. The spatial structure of the salt formed by the vinpocetine and D-tartaric acid is as shown in picture 1.
Owner:JIANGSU QINGJIANG PHARMA
A method for detecting the amount of carbon dioxide absorbed by ethanolamine
ActiveCN106370776BSmall source restrictionsEasy to transportChemical analysis using titrationCo2 absorptionTurbidity
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Poly(ether ketone ketone) polymer powder having a low volatiles content
A PEKK polymer powder, having a d0.9-value less than 150 μm, wherein the PEKK polymer has a Td(1%) of at least 500° C., as measured by thermal gravimetric analysis according to ASTM D3850, heating from 30° C. to 800° C. under nitrogen using a heating rate of 10° C. / min. A method for producing a PEKK powder for the use in a method for manufacturing a 3D object, in which the PEKK has a Td(1%) of at least 500° C., as measured by thermal gravimetric analysis according to ASTM D3850, heating from 30° C. to 800° C. under nitrogen using a heating rate of 10° C. / min, and the powder has been manufactured by grinding from a coarser powder and has a d0.9-value less than 150 μm, as measured by laser scattering in isopropanol.
Owner:SOLVAY SPECIALTY POLYMERS USA LLC
Heat preservation cover and method of using same to shorten filter paper ashing time in gravimetric analysis method
InactiveCN109269935AReduced ashing timePrevent splashWeighing by removing componentBiochemical engineeringProduct gas
The invention provides a heat preservation cover and a method of using the same to shorten filter paper ashing time in a gravimetric analysis method. The heat preservation cover includes a cover body.The cover body is a hollow structure, one end thereof is provided with a first hole, and the other end is provided with a second hole. An area of the first hole is smaller than an area of the secondhole. Handles are also arranged on the cover body. By using the heat preservation cover, which is provided by the invention, in conjunction with a heating device, heating efficiency of a filter paperashing process in the gravimetric analysis method can be greatly improved, the ashing time of filter paper can be shortened, a case where the filter paper burns, effective element loss is caused, andaccuracy of a measurement result is impacted is effectively avoided, and at the same time, a case where gas generated by a heating process quickly overflows and causes a blast and sample splashing isalso prevented.
Owner:CHENGDE JIANLONG SPECIAL STEEL
Analysis method for calculating liquid weight
InactiveCN102539268AWeighing apparatus for materials with special property/formWeighing by absorbing componentGas phaseSolvent
An analysis method for calculating liquid weight comprises determining a percentage content a% of a component A in to-be-determined liquid material by a chemical analysis method; adding a solvent with a weight W into the liquid material, and determining the percentage content b% of the component A; and calculating the weight of the to-be-determined liquid material according to a formula. The chemical analysis method comprises volumetry, gravimetry, visible absorptiometry, ultraviolet absorptiometry, gas and liquid chromatography, atom absorption spectrophotometry, potentiometry and molecular fluorescence analysis. The analysis method in the invention adopts the chemical analysis method to directly calculate out the weight of liquid material instead of weighing and measuring a volume, and can be used for determining a weight of liquid during chemical production and batching and in irregular containers.
Owner:NANKAI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com