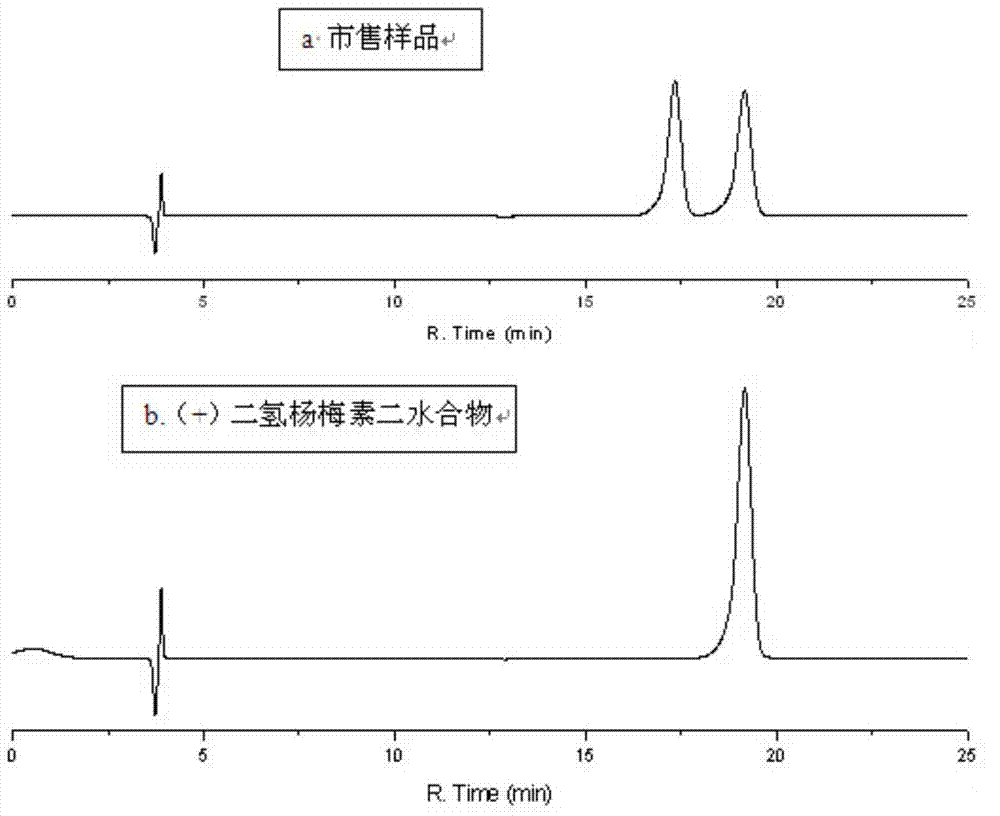
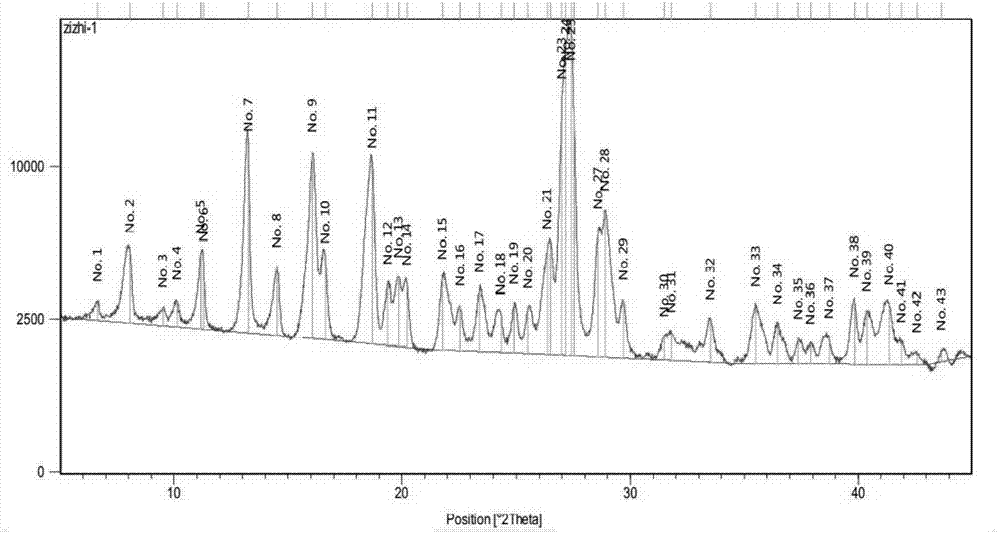
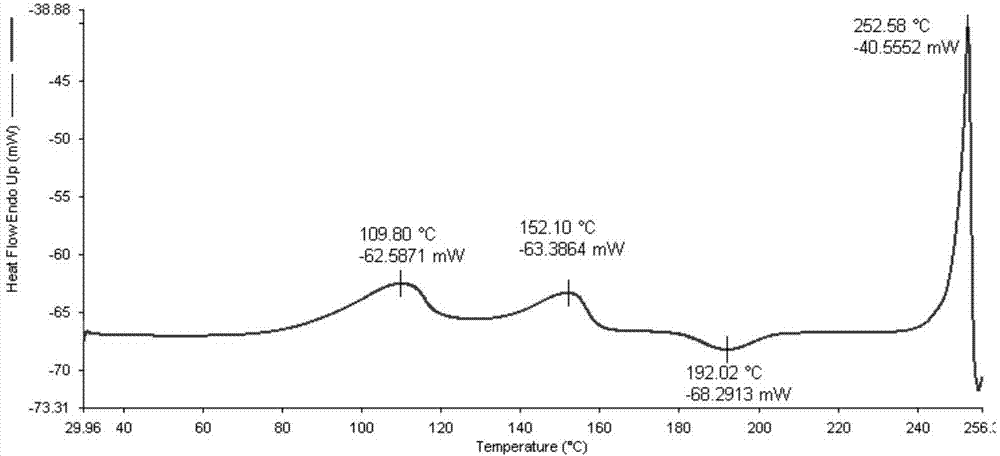
Five different crystalline form substances of dihydromyricelin
A technology of dihydromyricetin and d-dihydromyricetin, which is applied in the field of medicine and can solve problems such as inability to reveal the crystal structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] The preparation of embodiment 1 D-dihydromyricetin dihydrate
[0077] Take about 200mg of D-dihydromyricetin raw material, which can be anhydrous or monohydrate, dissolve it in 2ml of 90-100°C aqueous solution, filter with heat preservation, remove the filter residue, and cool the solution naturally to room temperature to precipitate crystals. Get D-dihydromyricetin dihydrate.
Embodiment 2
[0078] The preparation of embodiment 2 D-dihydromyricetin anhydrate
[0079] Take about 200 mg of D-dihydromyricetin raw material, which can be dihydrate or monohydrate, and place it at a temperature of 160 ° C for 1 hour to obtain dihydromyricetin anhydrate.
Embodiment 3
[0080] The preparation of embodiment 3 D-dihydromyricetin monohydrate
[0081] Take about 200 mg of D-dihydromyricetin raw material, which can be dihydrate or anhydrous, dissolve in high-concentration ethanol solution, volatilize slowly until crystallization, and obtain D-dihydromyricetin monohydrate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com