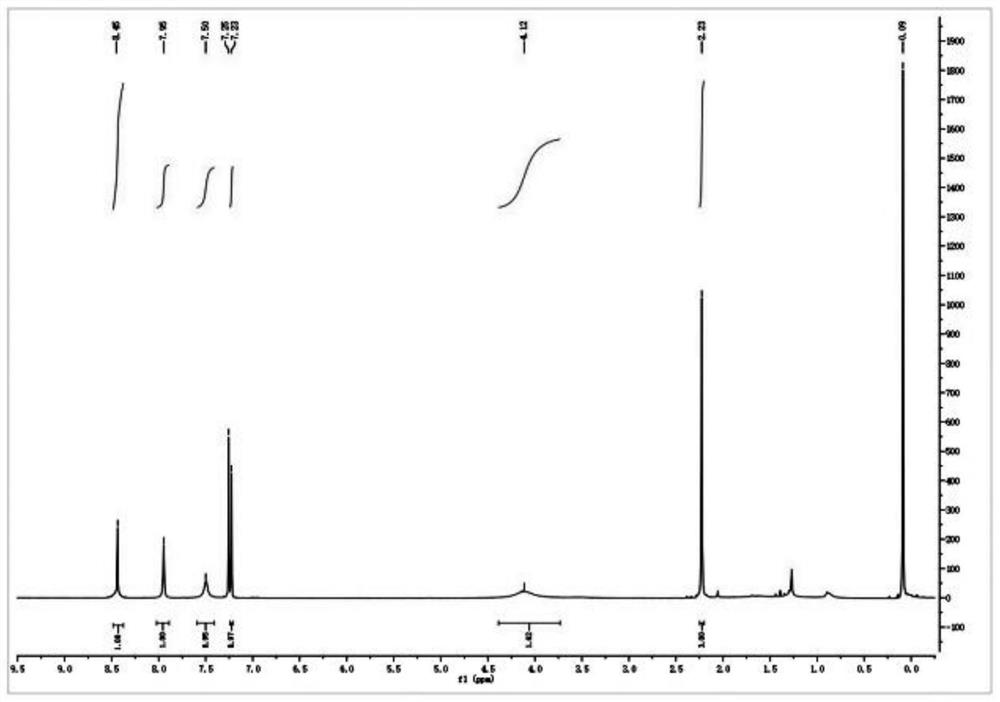
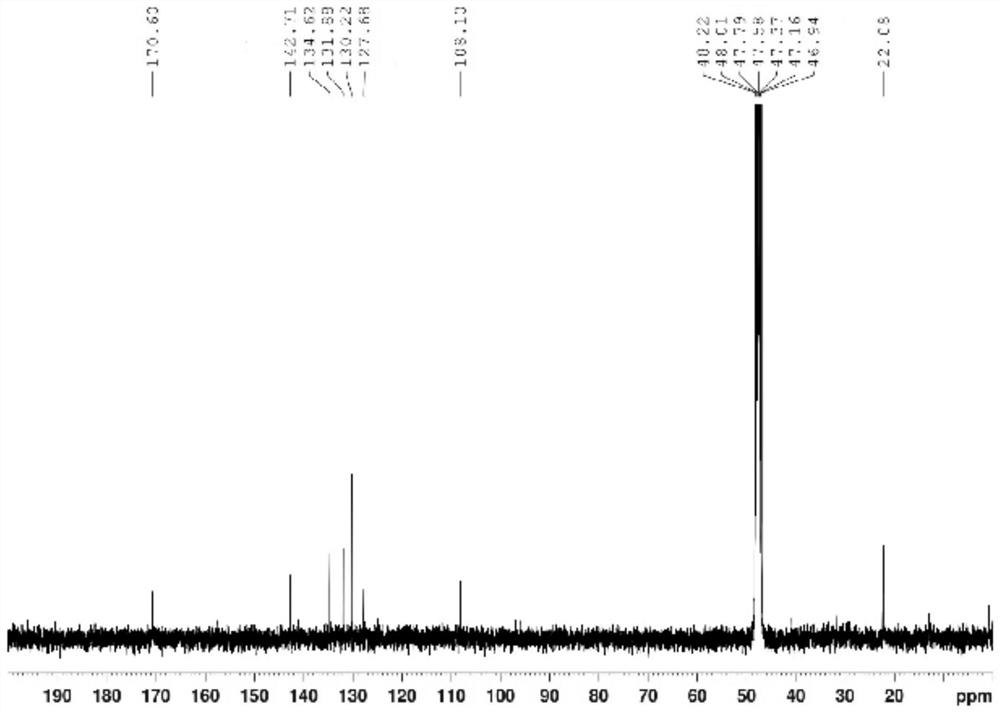
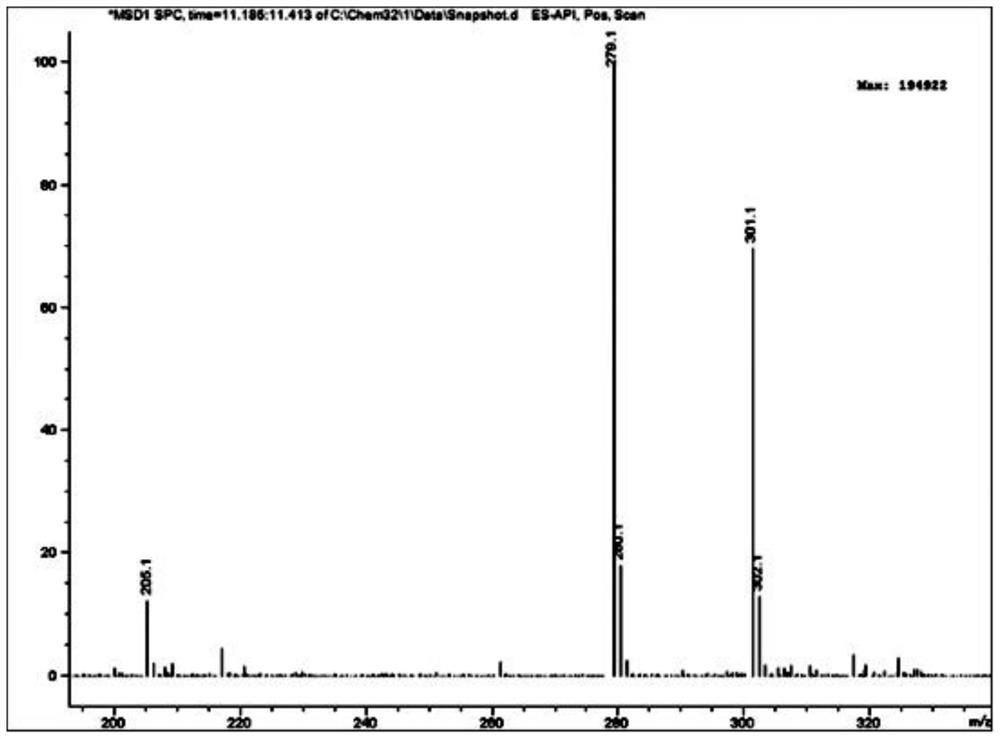
Preparation method of sulfonyl carfentrazone-ethyl
A technology of sulfentrazone and acetamide, which is applied in the field of preparation of sulfentrazone, can solve the problems of low yield, instability, unfavorable continuous improvement of the production process of sulfentrazone high-efficiency herbicide, etc., and achieve High-yield, easy-to-implement effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1. Add 64.80 g (0.40 mol) of 2,4-dichloroaniline and 300 ml of concentrated sulfuric acid to a 500 ml round-bottomed flask, and cool in an ice-water bath; add dropwise a mixture of 160 ml of concentrated sulfuric acid and 16 ml of concentrated nitric acid at 0°C, and dropwise add After completion, the reaction was continued at the same temperature for 2 hours. Then reaction mixture is added in 1500ml ice-water mixture, filter out precipitation, in Virahol / water mixed solvent (the volume ratio of the two in Virahol / water mixed solvent is 3:1, embodiment 2 is the same) 66.24 g (0.32 mol) of 2,4-dichloro-5-nitroaniline were obtained by crystallization in , with a yield of 80%.
[0057] 2. Add 200ml of dichloromethane, 62.10g (0.30mol) of 2,4-dichloro-5-nitroaniline and 66.79g (0.66mol) of triethylamine into a 500ml round bottom flask, and stir evenly. At room temperature, 86.35 g (0.33 mol) of acetyl chloride was added dropwise to the flask, and after the dropwise additio...
Embodiment 2
[0082] 1. Add 72.90 g (0.45 mol) of 2,4-dichloroaniline and 320 ml of concentrated sulfuric acid into a 500 ml round bottom flask, and cool in an ice-water bath. A mixture of 170ml of concentrated sulfuric acid and 17ml of concentrated nitric acid was added dropwise at 0°C, and the reaction was continued for 2.5 hours at the same temperature after the dropwise addition. The reaction mixture was added to 1550 ml of ice-water mixture, the precipitate was filtered off, and crystallized in isopropanol / water mixed solvent to obtain 75.55 g (0.365 mol) of 2,4-dichloro-5-nitroaniline, with a yield of 81%.
[0083] 2. Add 210ml of chloroform, 51.75g (0.25mol) of 2,4-dichloro-5-nitroaniline and 51.35g (0.65mol) of pyridine into a 250ml round bottom flask, and stir evenly at room temperature. At room temperature, 91.58 g (0.35 mol) of acetyl chloride was added dropwise to the flask, and after the dropwise addition was completed, the reaction was continued at room temperature for 4.5 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com