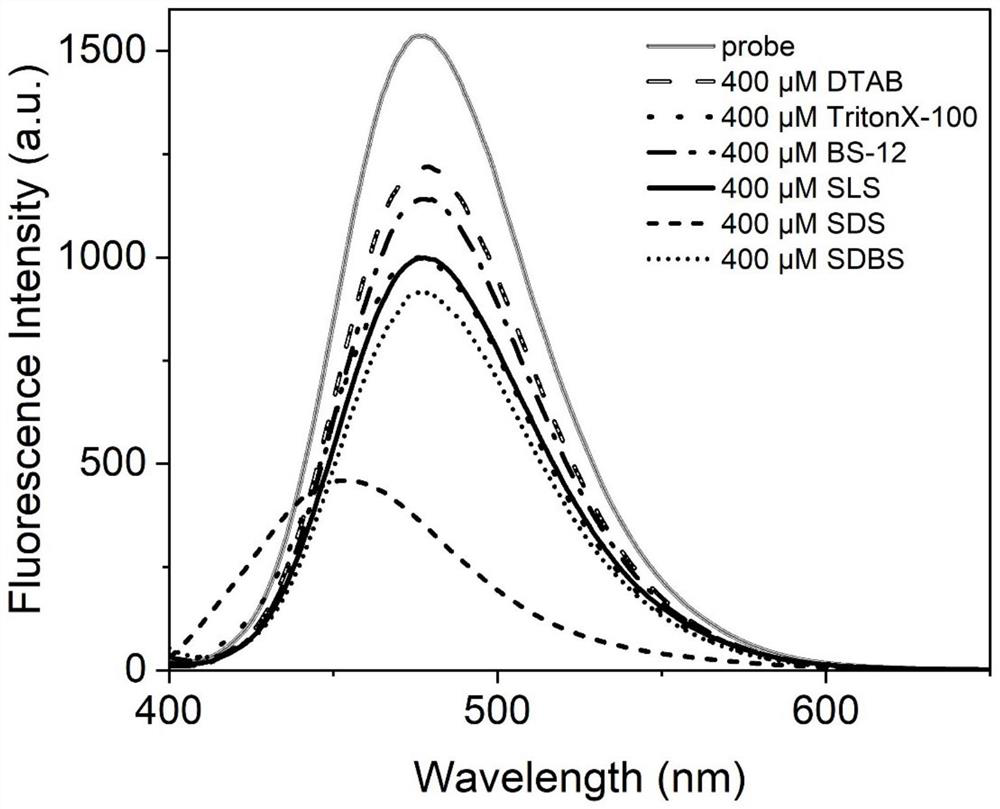
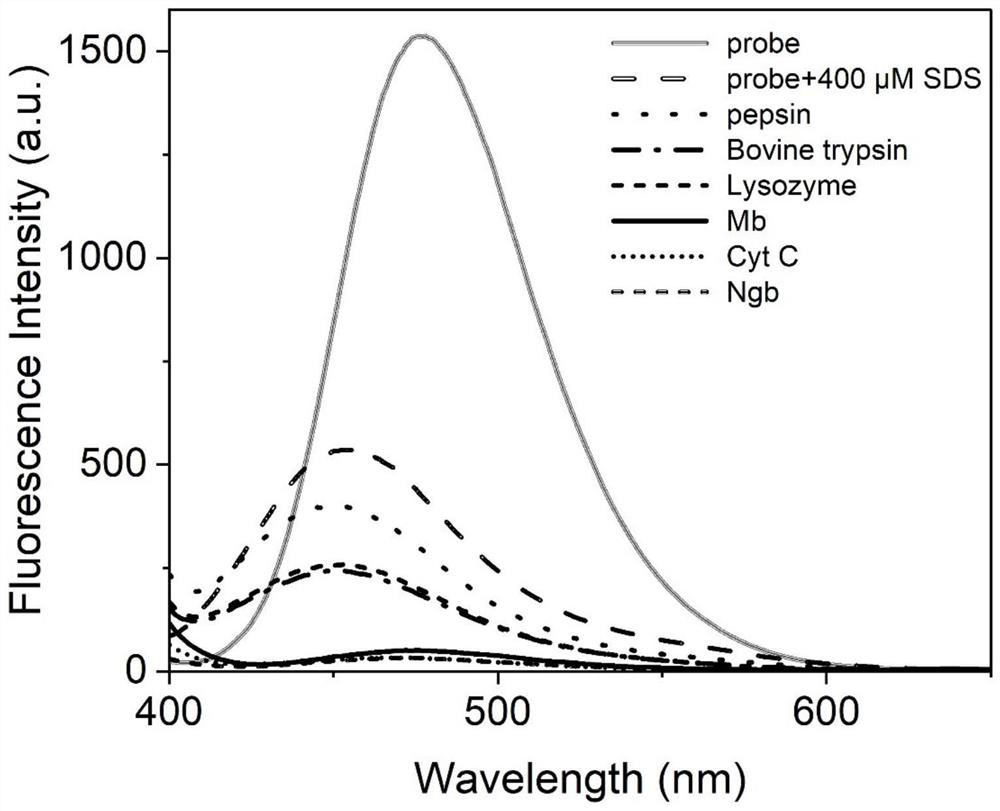
Fluorescent probe for identifying heme protein and application thereof
A technology of fluorescent probes and metalloproteins, applied in the field of fluorescent probes, can solve problems such as limited applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
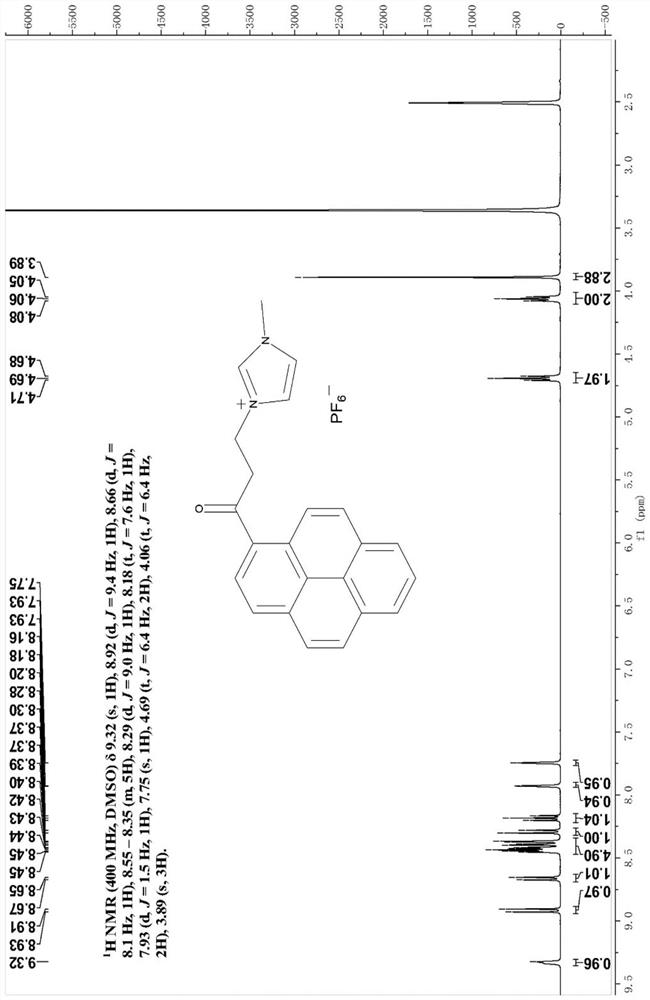
[0071] Example 1: Synthesis of Fluorescent Probes for Identifying Heme Proteins
[0072] Pyrene (4 g, 19.77 mmol) and 3-chloropropionyl bromide (2.04 g, 11.86 mmol) were dissolved in 40 mL of anhydrous DCM, cooled to 10 °C under nitrogen, and AlCl was added in portions within 20 min 3 (2.64 g, 19.77 mmol). The reaction mixture was darkened. The reaction mixture was allowed to slowly reach room temperature. The reaction mixture was stirred overnight. Cool excess aluminum chloride with crushed ice. The resulting aqueous phase was saturated with solid sodium chloride. 100-200 mL of fresh DCM was added and the layers were separated. The organic phase was washed with saturated sodium chloride. The mixture was dried over magnesium sulfate. Filter and concentrate in vacuo. The crude product was purified by silica gel (petroleum ether-DCM=7:3) column chromatography to obtain compound 3-chloro-1-pyrenylacetone.
[0073] A solution of 3-chloro-1-pyrenylacetone (0.51 g, 1.58 mmo...
Embodiment 2
[0077] Example 2: Fluorescent Probe Synthesis Method for Identifying Heme Proteins
[0078] Pyrene (4 g, 19.77 mmol) and 3-chloropropionyl bromide (2.72 g, 15.82 mmol) were dissolved in 40 mL of anhydrous DCM, cooled to 10 °C under nitrogen, and AlCl was added in portions within 20 min 3 (5.28 g, 39.54 mmol). The reaction mixture was darkened. The reaction mixture was allowed to slowly reach room temperature. The reaction mixture was stirred overnight. Cool excess aluminum chloride with crushed ice. The resulting aqueous phase was saturated with solid sodium chloride. 100-200 mL of fresh DCM was added and the layers were separated. The organic phase was washed with saturated sodium chloride. The mixture was dried over magnesium sulfate. Filter and concentrate in vacuo. The crude product was purified by silica gel (petroleum ether-DCM=7:3) column chromatography to obtain compound 3-chloro-1-pyrenylacetone.
[0079] A solution of 3-chloro-1-pyrenylacetone (0.51 g, 1.58 ...
Embodiment 3
[0083] Example 3: Synthesis of Fluorescent Probes for Identifying Heme Proteins
[0084] Pyrene (4 g, 19.77 mmol) and 3-chloropropionyl bromide (2.38 g, 13.84 mmol) were dissolved in 40 mL of anhydrous DCM, cooled to 10 °C under nitrogen, and AlCl was added in portions within 20 min 3 (3.96 g, 29.66 mmol). The reaction mixture was darkened. The reaction mixture was allowed to slowly reach room temperature. The reaction mixture was stirred overnight. Cool excess aluminum chloride with crushed ice. The resulting aqueous phase was saturated with solid sodium chloride. 100-200 mL of fresh DCM was added and the layers were separated. The organic phase was washed with saturated sodium chloride. The mixture was dried over magnesium sulfate. Filter and concentrate in vacuo. The crude product was purified by silica gel (petroleum ether-DCM=7:3) column chromatography to obtain compound 3-chloro-1-pyrenylacetone.
[0085] A solution of 3-chloro-1-pyrenylacetone (0.51 g, 1.58 mmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com