A kind of fluoroboron dipyrrole fluorescent probe, its preparation method and application in gold ion detection
A fluoroboron dipyrrole and fluorescent probe technology is applied in the field of fluorescence sensing and detection, which can solve the problems of expensive instrument cost, complex pretreatment and the like, and achieve the effects of accurate quantification, high sensitivity and high selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
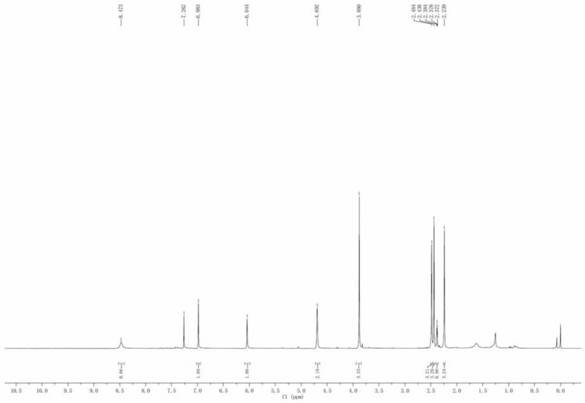
[0025] The compound 1,5,7-trimethyl-3-chloro-2-carboxylate methyl fluoroborodipyrrole (0.4g, 1.4mmol) was weighed and dissolved in dry tetrahydrofuran (20ml), and propargylamine (0.1g, 1.8mmol), heated to 50 degrees under nitrogen atmosphere and reacted for 2 hours; after the reaction, the solvent was distilled off under reduced pressure to an oily substance, purified by silica gel column chromatography, and ethyl acetate and petroleum ether=1:5 (v / v) Separation as an eluent to obtain a red solid fluorescent probe (yield 51%, purity 98%), the H NMR spectrum is as follows Figure 5 . The NMR data are as follows: 1 H NMR (400MHz, CDCl 3 ): δ8.47(s, 1H), 6.98(s, 1H), 6.05(s, 1H), 4.69(s, 2H), 3.88(s, 3H), 2.49(s, 3H), 2.44(s, 3H), 2.38(s, 1H), 2.24(s, 3H). 13 C NMR (100MHz, CDCl 3 ):δ166.4,157.9,150.2,146.3,135.7,131.2,130.9,117.6,115.8,107.7,79.3,72.7,51.6,34.6,14.4,12.2,11.1.
Embodiment 2
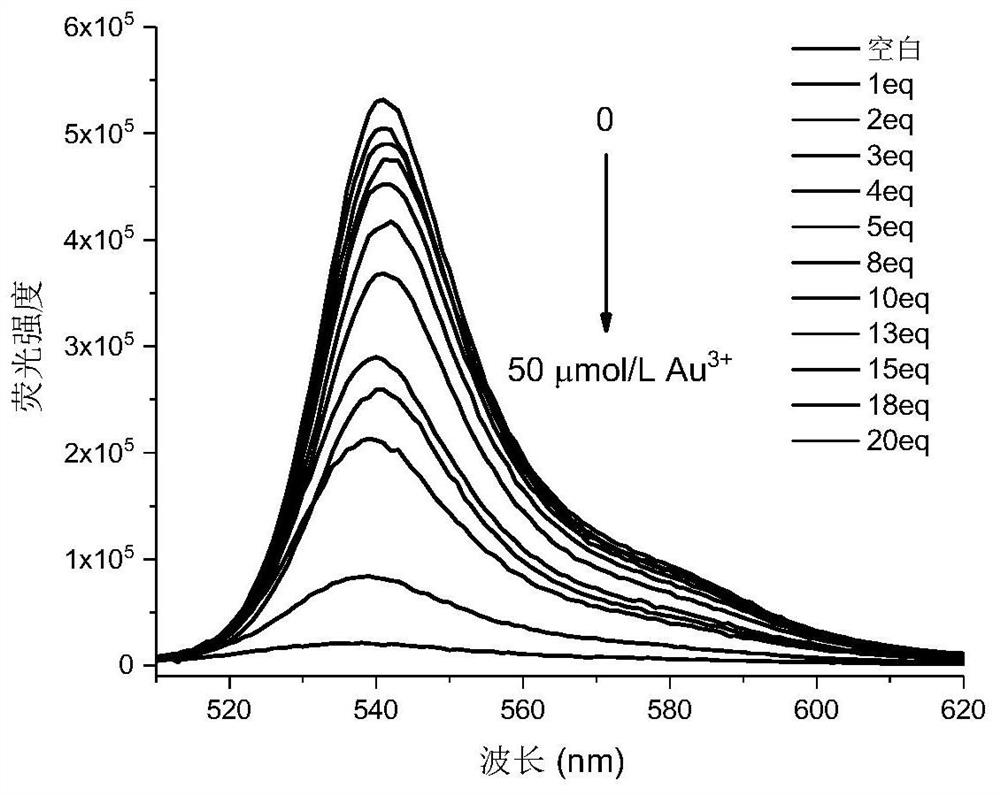
[0027] Fluorescence emission spectra of fluorescent probes in response to different gold ions: The probes were dissolved in ethanol / PBS buffer solution (v / v, 1 / 1, pH 7.0) to prepare a solution with a concentration of 2.5 μmol / L, and then dripped Add 0-50μmol / L aqueous solution of gold ions, and after equilibration, measure the fluorescence emission spectrum. The results are shown in figure 1 .
Embodiment 3
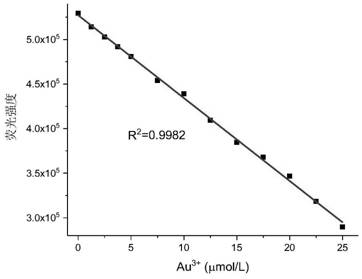
[0029] Fluorescence ratio change of fluorescent probe at 540nm (F / F 0 ) with different concentrations of gold ions: the probe was dissolved in ethanol / PBS buffer solution (v / v, 1 / 1, pH 7.0) to prepare a solution with a concentration of 2.5 μmol / L (μM), and then Add dropwise an aqueous solution of 0-25μmol / L gold ions, and after equilibration, measure the fluorescence emission spectrum, and plot the fluorescence intensity at 540 nm with the corresponding gold ion concentration data. The results are shown in figure 2 .
[0030] Depend on figure 2 It can be seen that the fluorescence value of the probe at 540nm will change linearly with the concentration of gold ions, and the limit of detection (LOD) of the probe to gold ions is calculated to be 180nM (36ppb). Quantification of gold ions in environmental water samples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com