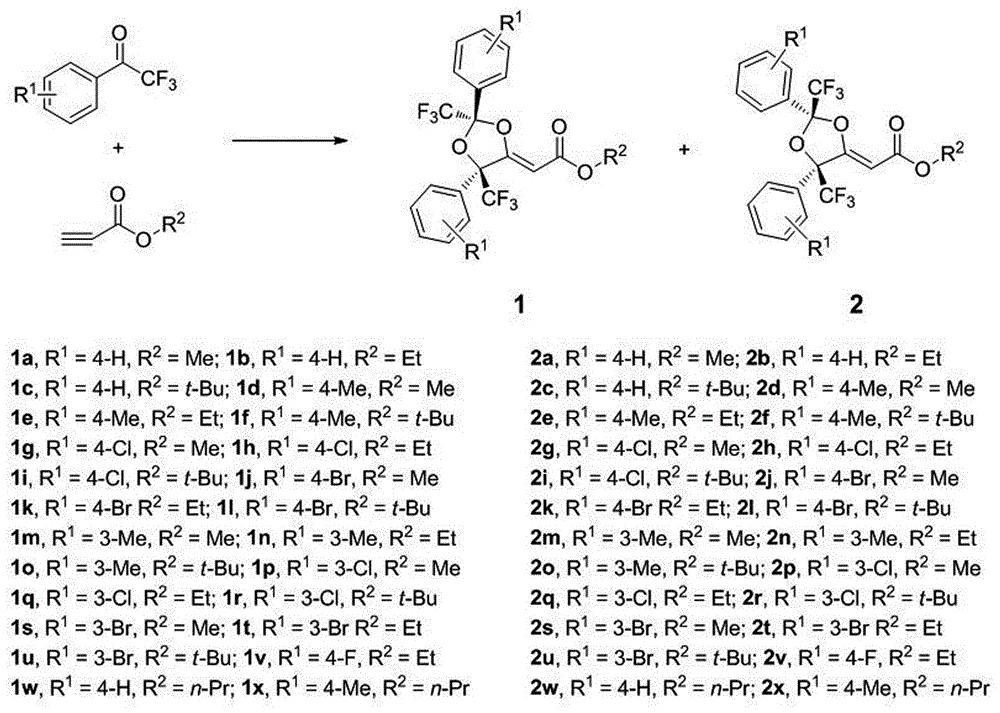Method for preparing trifluoromethyl functionalized 1,3-dioxolane
A technology of trifluoromethyl and dioxopentacycline, which is applied in the field of preparation of trifluoromethyl functionalized 1,3-dioxopentacycline, can solve problems such as difficulties in the synthesis process and inconformity with the principle of atom economy, and achieve The effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] 2,2,2-Trifluoroacetophenone (1.0mmol), ethyl propiolate (0.5mmol), AgF (0.05mmol), K 2 CO 3 (0.1 mmol) and DMSO (1 mL) were added to a Schrink flask, and the reaction was stirred at room temperature for 24 h, followed by TLC. After the reaction was completed, 15 mL of saturated brine was added to quench the reaction, and the reaction mixture was extracted with dichloromethane (15 mL×3). The organic phases were combined and concentrated using a rotary evaporator to obtain a crude product, which was obtained by column chromatography. The eluent used in column chromatography was petroleum ether: ethyl acetate (100:1), and the structure of the product was identified by NMR and high-resolution mass spectrometry. The separation yield of 1a reaches 43%, and the separation yield of 2a reaches 45%.
Embodiment 2
[0017] 2,2,2-Trifluoroacetophenone (1.0mmol), tert-butyl propiolate (0.5mmol), Ag 2 CO 3 (0.05 mmol), KOH (0.1 mmol) and DMA (1 mL) were added to a Schlink flask, and the reaction was stirred at room temperature for 24 h, followed by TLC. After the reaction was completed, 15 mL of saturated brine was added to quench the reaction, and the reaction mixture was extracted with dichloromethane (15 mL×3). The organic phases were combined and concentrated using a rotary evaporator to obtain a crude product, which was obtained by column chromatography. The eluent used in column chromatography was petroleum ether: ethyl acetate (100:1), and the structure of the product was identified by NMR and high-resolution mass spectrometry. The separation yield of 1a was 41%, and the separation yield of 2a was 42%.
Embodiment 3
[0019] 1-(4'-tolyl)-2,2,2-trifluoroethanone (1.0mmol), tert-butyl propiolate (0.5mmol), AgOOCH 2 CH 3 (0.05 mmol), NaHCO 3 (0.1 mmol) and DMA (1 mL) were added into a Schlinker bottle, and the reaction was stirred at room temperature for 24 h, and the reaction was tracked by thin-layer chromatography. After the reaction was completed, 15 mL of saturated brine was added to quench the reaction, and the reaction mixture was extracted with dichloromethane (15 mL × 3) to extract the reaction product. The organic phases were combined and concentrated using a rotary evaporator to obtain a crude product. The eluent used in column chromatography was petroleum ether: ethyl acetate (100:1), and the structure of the product was identified by NMR and high-resolution mass spectrometry. The separation yield of 1f reaches 40%, and the separation yield of 2f reaches 45%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com