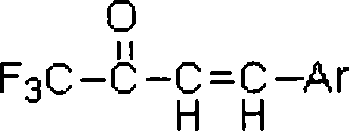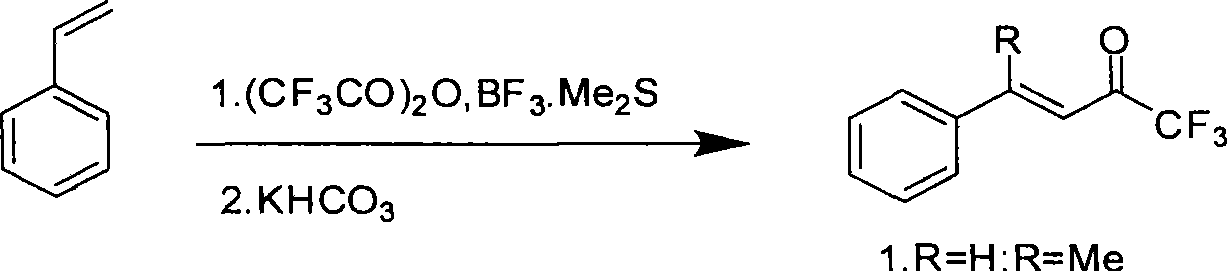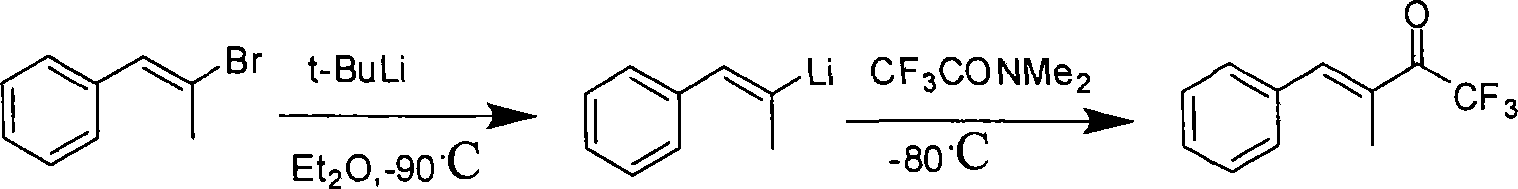Fluorine-containing alpha, beta-unsaturated ketone and synthetic method thereof
A synthetic method and unsaturated technology, applied in the field of fluorine-containing α, can solve the problems of inconvenient operation, average yield, and expensive raw materials, and achieve the effects of high selectivity, less by-products, and less pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0027] Example 1: Preparation of 1,1,1-trifluoro-4-(4-methyl-phenyl)-but-3-en-2-one
[0028] Add 1,1,1,5,5,5-hexafluoro-3-(4-methyl-styryl)-pentane-2,4-dione (310mg, 1mmol) in a 5ml eggplant-shaped bottle, Tetrahydrofuran (2ml), K 3 PO 4 ·3H 2 O (399mg, 1-5mmol), add a magnetic stirrer, under stirring, react at room temperature to 1,1,1,5,5,5-hexafluoro-3-(4-methyl-styryl)- Pentane-2,4-dione reacted completely, separated by column chromatography (developing solvent: ethyl acetate:petroleum ether=4:1), to obtain 1,1,1-trifluoro-4-(4- Methyl-phenyl)-but-3-en-2-one, the structure of the compound is:
[0029]
[0030] Molecular formula: C 11 h 9 f 3 o
[0031] Chinese name: 1,1,1-trifluoro-4-p-methylphenyl-3-en-2-one
[0032] English name: 1,1,1-trifluoro-4-p-tolybut-3-en-2-one
[0033] Molecular weight: 214.06
[0034] Appearance: light yellow liquid
[0035] Proton NMR spectrum (500MHz, CDCl 3 , internal standard: TMS): 2.42 (s, 3H, CH 3 ), 6.98 (d, J=16, 1H, CH...
example 2
[0037] Example 2: Preparation of 1,1,1-trifluoro-4-(4-methoxy-phenyl)-but-3-en-2-one
[0038] Add 1,1,1,5,5,5-hexafluoro-3-(4-methoxy-styryl)-pentane-2,4-dione (326mg, 1mmol) in a 5ml eggplant-shaped flask , tetrahydrofuran (2ml), K 3 PO 4 ·3H 2 O (532mg, 2mmol), add a magnetic stirrer, under stirring, react at room temperature to 1,1,1,5,5,5-hexafluoro-3-(4-methoxy-styryl)-pentane The reaction of alkane-2,4-dione was complete, and it was separated by column chromatography (developing solvent: ethyl acetate:petroleum ether=4:1) to obtain 1,1,1-trifluoro-4-(4-methoxy Base-phenyl)-but-3-en-2-one, the structure of the compound is:
[0039]
[0040] Molecular formula: C 11 h 9 f 3 o 2
[0041] Chinese name: 1,1,1-trifluoro-4-p-methoxyphenyl-3-en-2-one
[0042] English name: 1,1,1-trifluoro-4-(4-methoxyphenyl)but-3-en-2-one
[0043] Molecular weight: 230.06
[0044] Appearance: light yellow liquid
[0045] Proton NMR spectrum (500MHz, CDCl 3 , internal standard: TM...
example 3
[0047] Example 3: Preparation of 1,1,1-trifluoro-4-(2-furyl)-but-3-en-2-one
[0048] Add 1,1,1,5,5,5-hexafluoro-3-(2-furyl-vinyl)-pentane-2,4-dione (286mg, 1mmol), tetrahydrofuran into a 5ml eggplant-shaped bottle (2ml), K 3 PO 4 ·3H 2 O (798mg, 3mmol), add a magnetic stirrer, under stirring, react at room temperature to 1,1,1,5,5,5-hexafluoro-3-(2-furyl-vinyl)-pentane- The reaction of 2,4-diketone was complete, and it was separated by column chromatography (developing solvent: ethyl acetate:petroleum ether=4:1) to obtain 1,1,1-trifluoro-4-(4-methyl-benzene Base)-but-3-en-2-one, the structure of the compound is:
[0049]
[0050] Molecular formula: C 8 h 5 f 3 o 2
[0051] Chinese name: 1,1,1-trifluoro-4-(2-furyl)-3-en-2-one
[0052] English name: 1,1,1-trifluoro-4-(furan-2-yl)but-3-en-2-one
[0053] Molecular weight: 190.02
[0054] Appearance: light yellow liquid
[0055] Proton NMR spectrum (500MHz, CDCl 3 , internal standard: TMS): 6.57 (m, J=1.5, 3.5, 1H, f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com