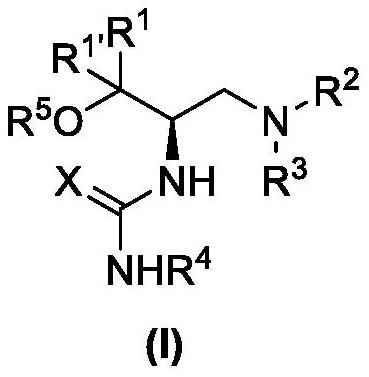
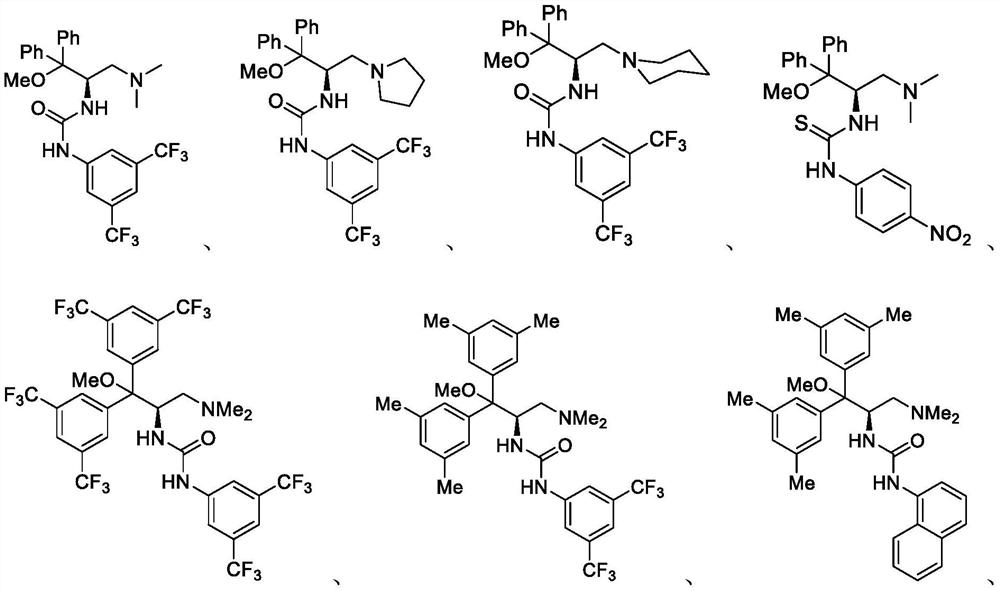
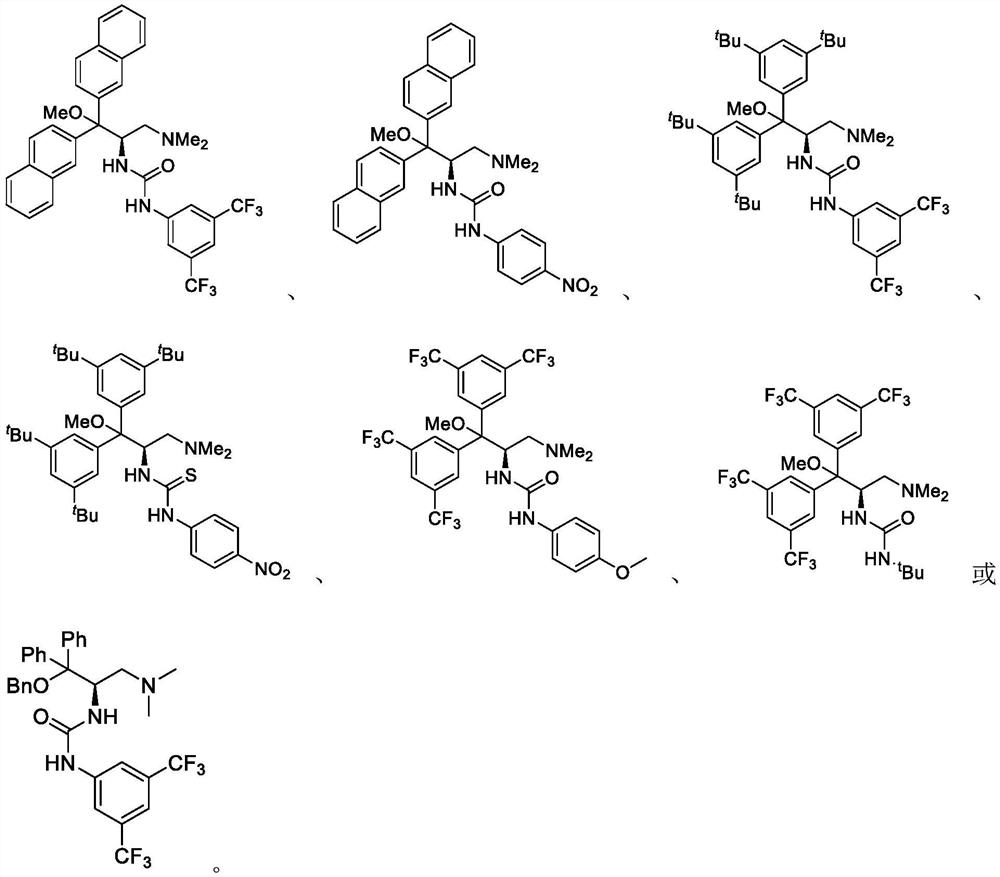
Method for catalyzing asymmetric Henry reaction of trifluoromethyl ketone
A technology of methyl and alkyl, which is applied in the field of catalyzing the asymmetric Henry reaction of trifluoromethyl ketone, which can solve problems such as complicated operation, low universality of trifluoromethyl ketone, and difficult preparation of catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0194] Put (D)-serine methyl ester hydrochloride (10.0g, 64mmol, 1.0equiv.,) into the three-necked flask, dissolve it in 150mL1,2-dichloroethane, add triethylamine (16.0g, 2.5equiv.,) , after which TrtCl (15.4 g, 1.0 equiv.) was added in portions. Then, the reaction system was heated and stirred in an oil bath at 50° C. for 12 hours. TLC detected that the reactant completely disappeared, and the reaction system was placed in an ice-water bath. When the internal temperature reached 10°C, the reaction was quenched by slowly adding saturated ammonium chloride aqueous solution dropwise. The chloroform layer was separated, washed with saturated brine, dried by adding anhydrous sodium sulfate, filtered to obtain the organic phase, evaporated to remove the solvent, and recrystallized in a mixed solvent of petroleum ether / ethyl acetate to obtain 22.38 g of a white solid (compound A). Yield 97%.
Embodiment 2
[0196] Dissolve 22.38g (62mmol, 1.0eq.) of the amino-protected product (Compound A) in dichloromethane, cool the system to 0°C, and drop triethylamine (2.5eq., 11.0mL) and MsCl (1.5 equiv., 3.7mL), the dropwise addition was completed, and the ice-salt bath was removed, and the system naturally returned to room temperature and stirred for 24 hours. TLC detected that the reactant completely disappeared, and added deionized water to quench the reaction, and then separated to obtain a dichloromethane layer. Wash with saturated brine, add anhydrous sodium sulfate to dry, distill off the solvent, and recrystallize in a mixed solvent of n-hexane / methyl tert-butyl ether to obtain 20.9 g of white solid (compound B), yield 98%.
Embodiment 3
[0198] Under the protection of argon, add 3.0g (125mmol, 2.1eq.) of metal magnesium and 20.0mL of THF into a three-necked flask, add 1 grain of iodine to catalyze, and then slowly add a THF solution of 15.7g (125mmol, 2.1eq.) of bromobenzene (40.0mL) to keep the system in a slightly boiling state. After the dropwise addition was completed, reflux was continued for 1.5 hours. Then the system was cooled to 0°C in an ice-water bath, and a THF solution (50.0 mL) of 20.9 g (61 mmol, 1 eq.) of the ring-closing product (compound B) was slowly added dropwise. After the addition was complete, the system was returned to room temperature and continued to stir for 4 Hour. After TLC detects that the reactant completely disappears, quench the reaction by adding saturated ammonium chloride aqueous solution under ice-water bath cooling, extract three times with ethyl acetate, separate the organic layer, wash with saturated brine, dry over anhydrous sodium sulfate, and evaporate the solvent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com