A kind of visible light catalyzed preparation method of α-aryl-β-trifluoromethyl ketone compound
A technology for the catalytic preparation of trifluoromethyl ketones, which is applied in the field of visible light catalytic preparation of α-aryl-β-trifluoromethyl ketones, which can solve the problems of unstable chemical properties, high price, high reaction temperature, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
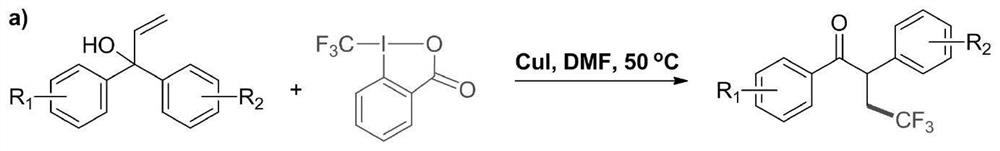
[0027] A method for preparing 4,4,4-trifluoro-1,2-diphenyl-1-butanone compound by visible light catalysis, the method is represented by the following reaction formula:
[0028]
[0029] Preparation of 4,4,4-trifluoro-1,2-diphenyl-1-butanone: Add 2.1 g of 1,1-diphenyl to a 200 ml dry single-necked bottle in the presence of atmospheric pressure of air Phenyl-2-propen-1 alcohol (10 mmol), 3.12 g of sodium trifluoromethylsulfinate (20 mmol), 0.158 g of 1,2,3,4-tetrakis(carbazol-9-yl )-4,6-dicyanobenzene (0.2 mmol) and 80 ml of 1,2-dichloroethane. Place the reaction solution at room temperature under 18-watt blue LED light irradiation (the distance between the reaction bottle and the light source is about 10-15 cm) until 1,1-diphenyl-2-propen-1 alcohol completely disappears (TLC track). After the reaction was completed, the 1,2-dichloroethane solvent was evaporated by rotary evaporation, and the obtained crude product was separated by column chromatography (eluent: n-hexane / et...
Embodiment 2
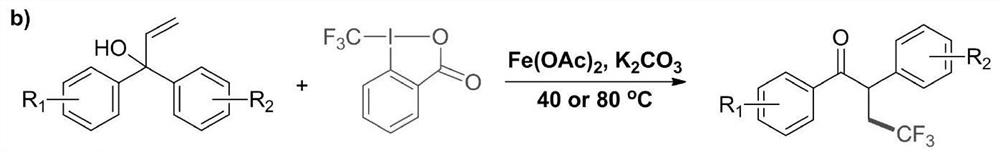
[0033] A method for preparing 4,4,4-trifluoro-2-methyl-1,2-bis(4-methyl-phenyl)-1-butanone compound by visible light catalysis, the method is represented by the following reaction formula :
[0034]
[0035]The preparation of 4,4,4-trifluoro-2-methyl-1,2-di(4-methyl-phenyl)-1-butanone: In the presence of atmospheric pressure air, pour into a 200 ml dry Add 2.52 grams of 2-methyl-1,1-bis(4-methyl-phenyl)-2-propen-1-ol (10 mmol), 3.12 grams of trifluoromethylsulfinic acid successively in the single-necked bottle Sodium (20 mmol), 0.158 g of 1,2,3,4-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (0.2 mmol) and 80 ml of 1,2-dichloro ethane. At room temperature, place the reaction solution under an 18-watt blue LED light (the distance between the reaction bottle and the light source is about 10-15 cm) until 2-methyl-1,1-di(4-methyl-phenyl )-2-propen-1-ol disappeared completely (TLC tracking). After the reaction was completed, the 1,2-dichloroethane solvent was evaporated by rota...
Embodiment 3
[0039] A method for preparing 4,4,4-trifluoro-1-phenyl-2-(2-thienyl)-1-butanone compound by visible light catalysis, the method is represented by the following reaction formula:
[0040]
[0041] Preparation of 4,4,4-trifluoro-1-phenyl-2-(2-thienyl)-1-butanone: In the presence of atmospheric pressure air, add 2.16 g 1-phenyl-1-(2-thienyl)-2-propen-1-ol (10 mmol), 3.12 g of sodium trifluoromethylsulfinate (20 mmol), 0.158 g of 1,2 , 3,4-Tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (0.2 mmol) and 80 mL of 1,2-dichloroethane. At room temperature, place the reaction solution under an 18-watt blue LED light (the distance between the reaction bottle and the light source is about 10-15 cm) until 1-phenyl-1-(2-thienyl)-2-propene- The 1-ol disappeared completely (TLC trace). After the reaction was completed, the 1,2-dichloroethane solvent was evaporated by rotary evaporation, and the obtained crude product was separated by column chromatography (eluent: n-hexane / ethyl acetate=10:1-4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com