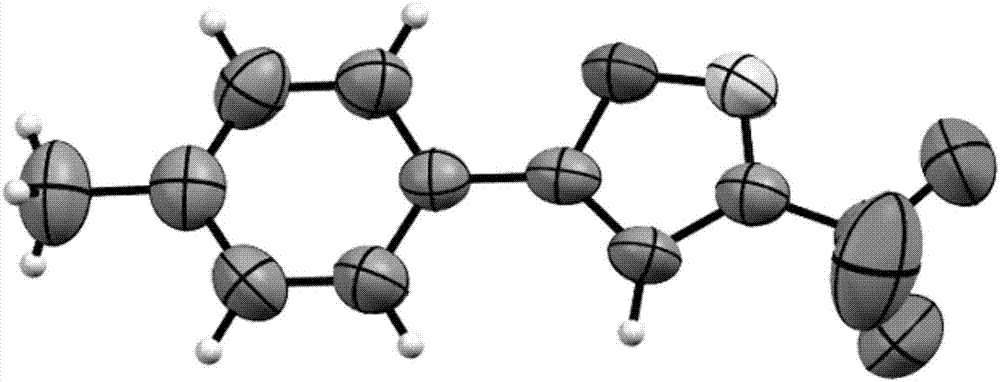
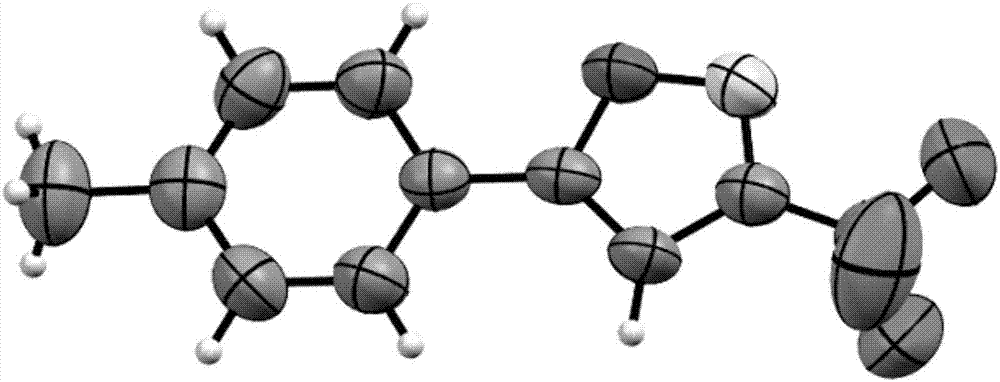
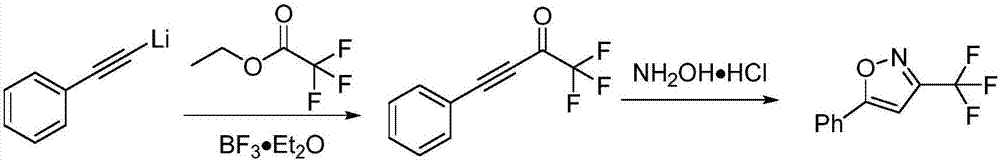
Method for preparing 3-trifluoromethylisooxazole compound by one-pot
A technology of isoxazole compound and trifluoromethyl, which is applied in the field of preparation of isoxazole compound, can solve the problems of low utilization rate of raw materials, lengthy reaction steps, harsh reaction conditions, etc., and achieve reduced production cost, simple operation, and side effects less responsive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Substrate: 4-Phenylphenylacetylene
[0034] product:
[0035] Product characterization data: pale yellow solid (60.7mg, 84% yield); mp: 128.5–129.5; 1 H NMR (400MHz, CDCl 3 )δ7.88(d, J=8.0Hz, 2H), 7.73(d, J=8.0Hz, 2H), 7.64(d, J=8.0Hz, 2H), 7.49(t, J=8.0Hz, 2H) ,7.42(d,J=8.0Hz,1H),6.77(s,1H); 13 C NMR (100MHz, CDCl 3 )δ172.5, 156.4 (q, J=38.0Hz), 144.4, 140.0, 129.4, 128.6, 128.2, 127.4, 126.8, 125.2, 120.1 (q, J=270.0Hz), 97.1; 19 F NMR (376MHz, CDCl 3 )δ-63.19(s,3F); 19 F{ 1 H}NMR (376MHz, CDCl 3)δ-63.19(s,3F); HRMS(ESI)m / z calcd for C 16 h 11 ONF 3 + [M+H] + 290.0787,found 290.0784.
Embodiment 2
[0037] Substrate: Phenylacetylene
[0038] product:
[0039] Product characterization data: pale yellow solid (46.7mg, 88% yield); mp: 44.4–45.2; 1 H NMR (400MHz, CDCl 3 )δ7.83–7.78(m,2H),7.53–7.50(m,3H),6.74(s,1H); 13 C NMR (100MHz, CDCl 3 )δ172.8, 156.4 (q, J=38.0Hz), 131.6, 129.6, 126.4, 120.1 (q, J=270Hz), 97.1; 19 FNMR (376MHz, CDCl 3 )δ-63.27(s,3F); 19 F { 1 H}NMR (376MHz, CDCl 3 )δ-63.27(s,3F); HRMS(ESI)m / zcalcd for C 10 h 7 ONF 3 + [M+H] + 214.0474,found 214.0471.
Embodiment 3
[0041] Substrate: 4-methylphenylacetylene
[0042] product:
[0043] Product characterization data: white solid (49.6mg, 87% yield); mp: 71.6–72.3; 1 H NMR (400MHz, CDCl 3 )δ7.69(d, J=8.0Hz, 2H), 7.30(d, J=8.0Hz, 2H), 6.68(s, 1H), 2.42(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ173.0, 156.3 (q, J = 38.0Hz), 142.2, 130.3, 126.3, 123.8, 120.1 (q, J = 270.0Hz), 96.5, 21.8; 19 F NMR (376MHz, CDCl 3 )δ-63.30(s,3F); 19 F{ 1 H}NMR (376MHz, CDCl 3 )δ-63.30(s,3F); HRMS(ESI)m / z calcd for C 11 h 9 ONF 3 + [M+H] + 228.0631, found 228.0628. [13]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com