Trifluoroacetyl substituted hydrazone derivative and synthesis method thereof
A technique for the synthesis of trifluoroacetyl groups, which is applied in the field of trifluoroacetyl substituted hydrazone derivatives and their synthesis, can solve problems such as blanks, and achieve the effects of simple operation, mild reaction conditions, and high product yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
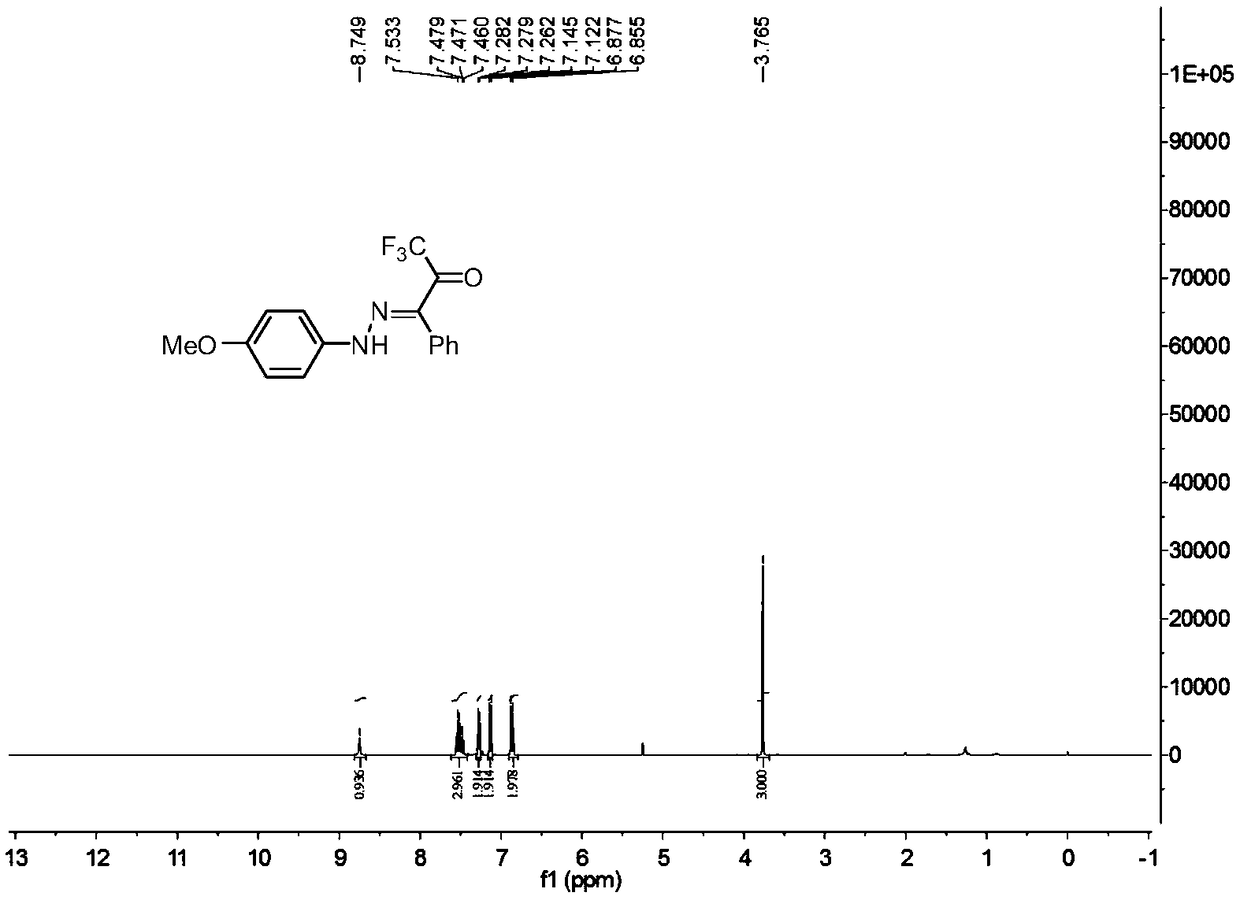
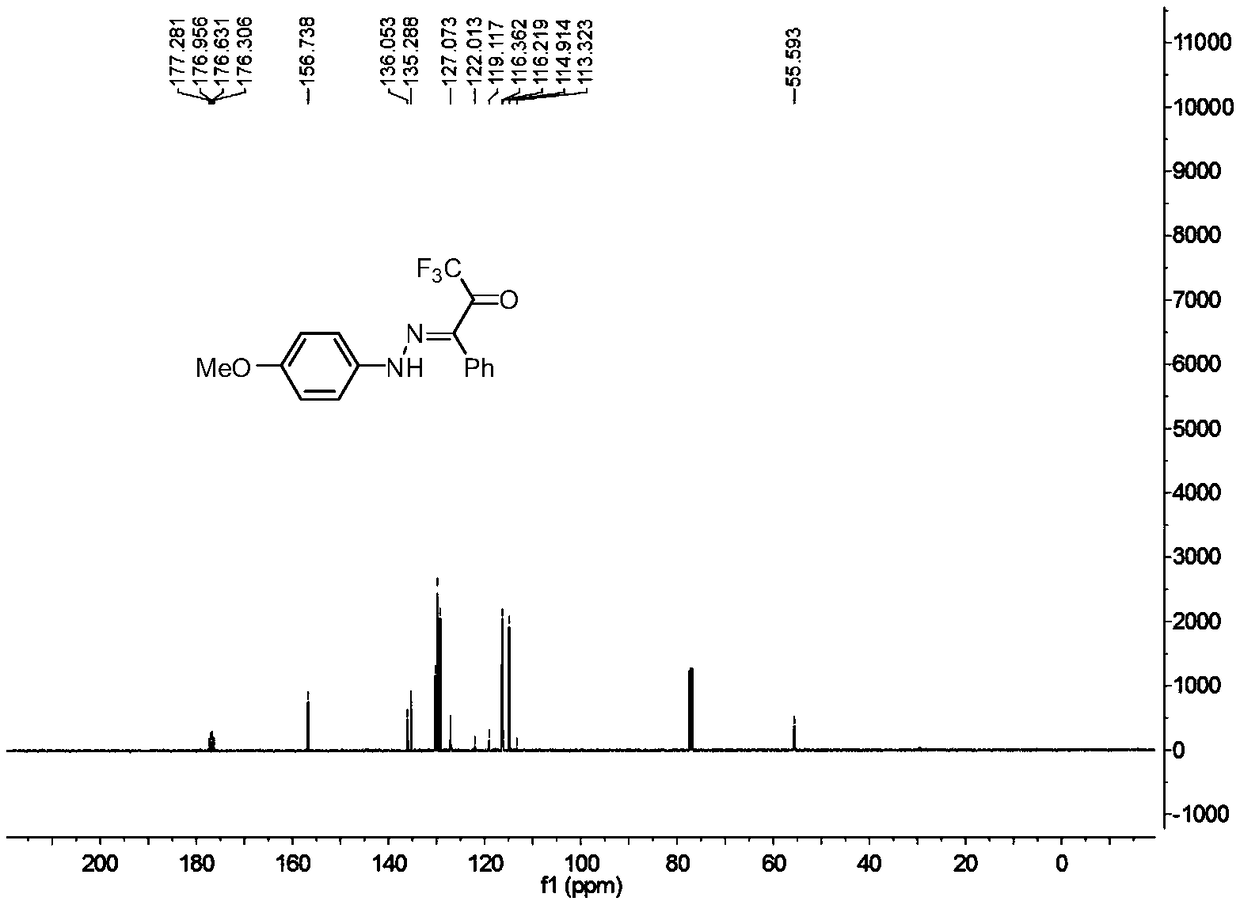
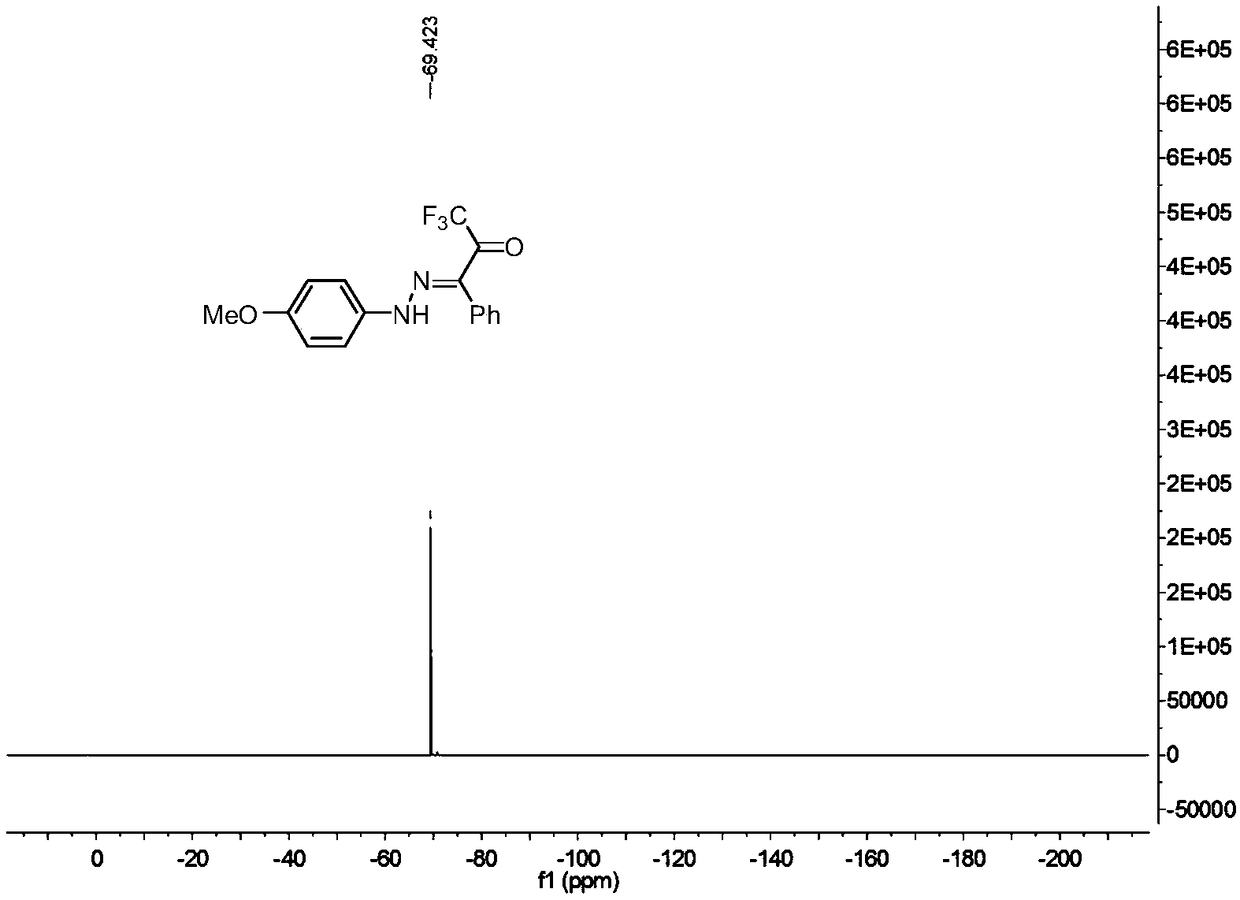
[0029] In a 25ml reaction flask, add 0.2mmol of p-methoxyphenyldiazonium tetrafluoroborate, 0.3mmol of triethylenediamine, 0.3mmol of 3-phenyl-1,1,1,-trifluoro Acetone, 2 ml of acetonitrile, and the reaction system was stirred at 25°C for 0.05 hour. Add water, extract the reaction solution with ethyl acetate, remove the solvent by rotary evaporation under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The column chromatography eluent used is petroleum ether: ethyl acetate with a volume ratio of 10:1 ester mixed solvent to obtain the product with a yield of 97%.
Embodiment 2
[0031] In a 25ml reaction flask, add 0.2mmol of p-methoxyphenyldiazonium tetrafluoroborate, 0.3mmol of triethylenediamine, 0.3mmol of 3-phenyl-1,1,1,-trifluoro Acetone, 2 ml of acetonitrile, and the reaction system was stirred at 25°C for 0.05 hour. Add water, extract the reaction solution with methylene chloride, remove the solvent by rotary evaporation under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The column chromatography eluent used is petroleum ether:ethyl acetate in a volume ratio of 10:1 ester mixed solvent to obtain the product with a yield of 97%.
Embodiment 3
[0033] In a 25 ml reaction flask, add 0.2 mmol p-methoxyphenyldiazonium hexafluorophosphate, 0.3 mmol potassium carbonate, 0.3 mmol 3-phenyl-1,1,1,-trifluoroacetone, 2 mL of acetonitrile, and the reaction system was stirred at 25°C for 0.05 hour. Add water, extract the reaction solution with ethyl acetate, remove the solvent by rotary evaporation under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The column chromatography eluent used is petroleum ether: ethyl acetate with a volume ratio of 10:1 ester mixed solvent to obtain the product with a yield of 64%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com