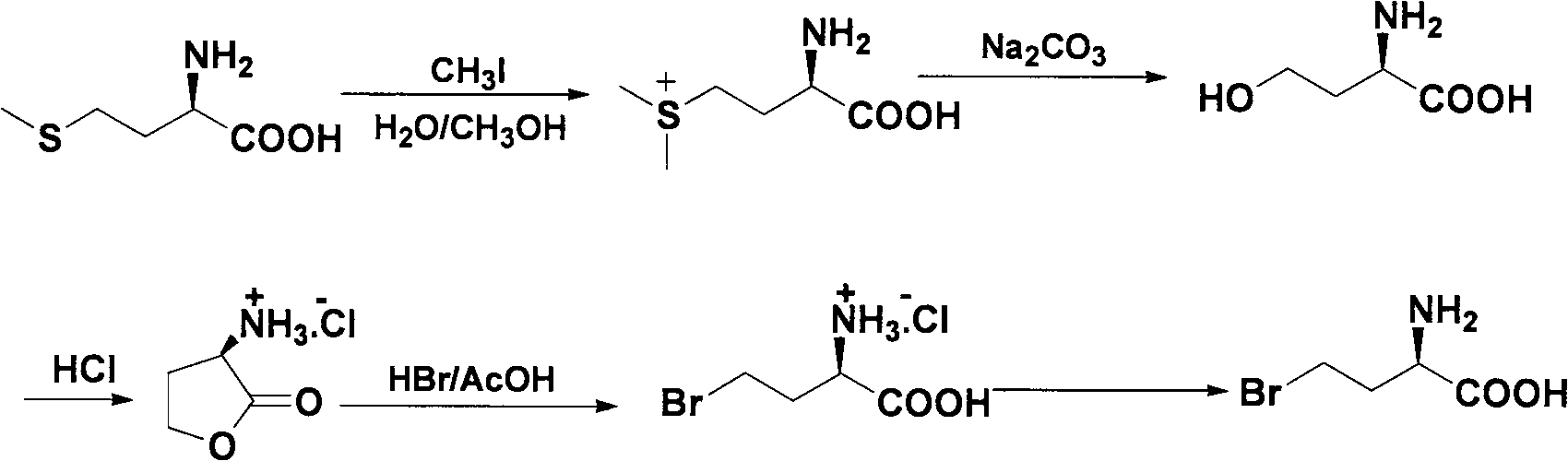
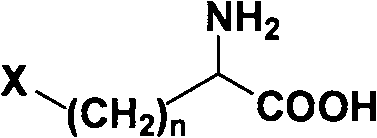
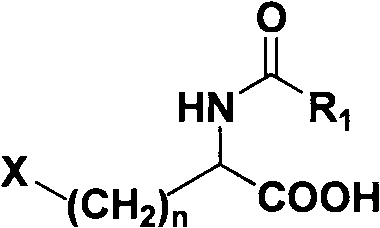
Method for splitting halogenated alpha-amino acid
A technology of amino acid and halogenation, which is applied in the field of production process of halogenated α-amino acid, to achieve the effect of simple operation, mild reaction conditions and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment one: the separation process of L-chloroalanine and D-chloroalanine
[0026] 1. Synthesis of N-phenylacetyl-DL-chloroalanine:
[0027] Add 123.5gDL-chloroalanine, 40gNaOH, and 500ml water into a 1000ml reaction flask, stir fully at 0°C, add 170g of phenylacetyl chloride dropwise under stirring, control pH=8-10 during the reaction, and temperature 0°C Next, after the completion of the dropwise addition, keep the reaction for 2 hours, then continue to stir the reaction at room temperature for 3 hours, stop the reaction, add concentrated hydrochloric acid to adjust the pH to 1-2, a large amount of white solid is precipitated, vacuum filtration to obtain 229.5g of white solid N -Phenylacetyl-DL-chloroalanine. The properties of the products obtained were determined by HPLC (High Performance Liquid Chromatography).
[0028] 2. Enzymatic hydrolysis of N-phenylacetyl-DL-chloroalanine:
[0029] Add 120.5g of N-phenylacetyl-DL-chloroalanine solid obtained in the prev...
Embodiment 2
[0035] Embodiment two: the separation process of L-bromoalanine and D-bromoalanine
[0036] 1. Synthesis of N-phenylacetyl-DL-bromoalanine:
[0037] Add 168gDL-bromoalanine, 40gNaOH, and 600ml water into a 1000ml reaction flask, stir fully at -5°C, add 170g of phenylacetyl chloride dropwise under stirring, control pH=8-9 during the reaction, and temperature -5 Below ℃, keep the reaction for 4 hours after the dropwise addition is completed, then continue to stir the reaction at room temperature for 5 hours, stop the reaction, add dilute sulfuric acid to adjust the pH to 1-2, a large amount of solids are precipitated, vacuum filtration, and 266g of white solid N- Phenylacetyl-DL-bromoalanine.
[0038] The properties of the products obtained were determined by HPLC (High Performance Liquid Chromatography).
[0039] 2. Enzymatic hydrolysis of N-phenylacetyl-DL-bromoalanine:
[0040] Add 143g of N-phenylacetyl-DL-bromoalanine solid obtained in the previous step into a 2000ml react...
Embodiment 3
[0045] Example 3: Resolution process of L-fluoroalanine and D-fluoroalanine
[0046] 1. Synthesis of N-phenylacetyl-DL-fluoroalanine:
[0047] Add 107.1gDL-fluoroalanine, 40gNaOH, and 450ml water into a 1000ml reaction flask, stir well at -2°C, add 170g of phenylacetyl chloride dropwise under stirring, control pH=8-9 during the reaction, temperature- Below 2°C, keep warm for 3 hours after the dropwise addition, then continue to stir and react at room temperature for 2 hours, stop the reaction, add concentrated hydrochloric acid to adjust the pH to 1-2, a large amount of white solid precipitates, and vacuum filter to obtain 209.5g of white Solid N-phenylacetyl-DL-fluoroalanine.
[0048] The properties of the products obtained were determined by HPLC (High Performance Liquid Chromatography).
[0049] 2. Enzymatic hydrolysis of N-phenylacetyl-DL-fluoroalanine:
[0050] Add 112.6g of N-phenylacetyl-DL-fluoroalanine solid obtained in the previous step into a 2000ml reaction flas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com