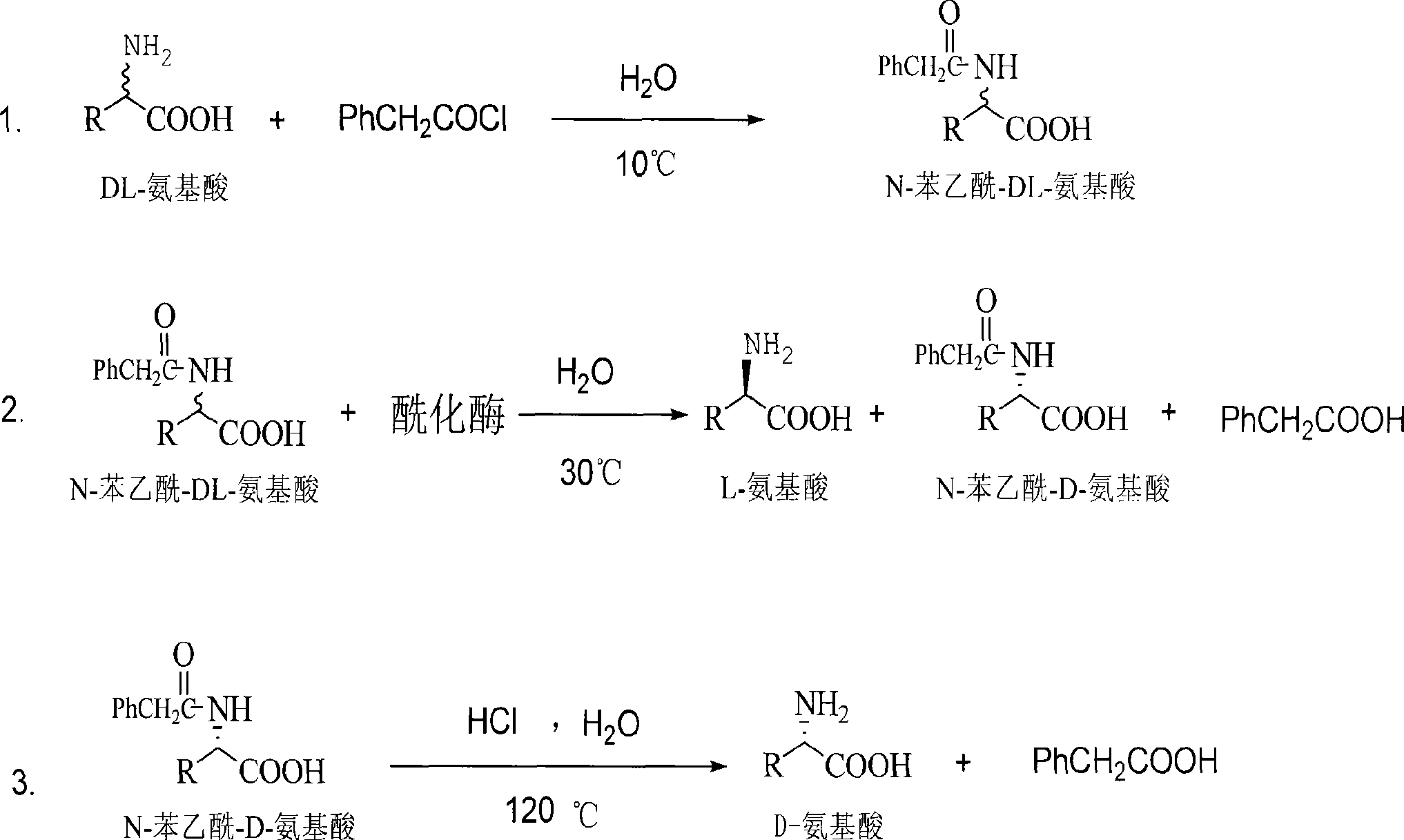Method for preparing D-aminophenol with immobilization penicillin acylated enzyme catalysis
An amino acid and methionine technology, which is applied in the field of D-amino acid production technology, can solve the problems of high preparation cost of hydantoin and limited large-scale application, and achieve the effects of low production cost, short splitting route and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
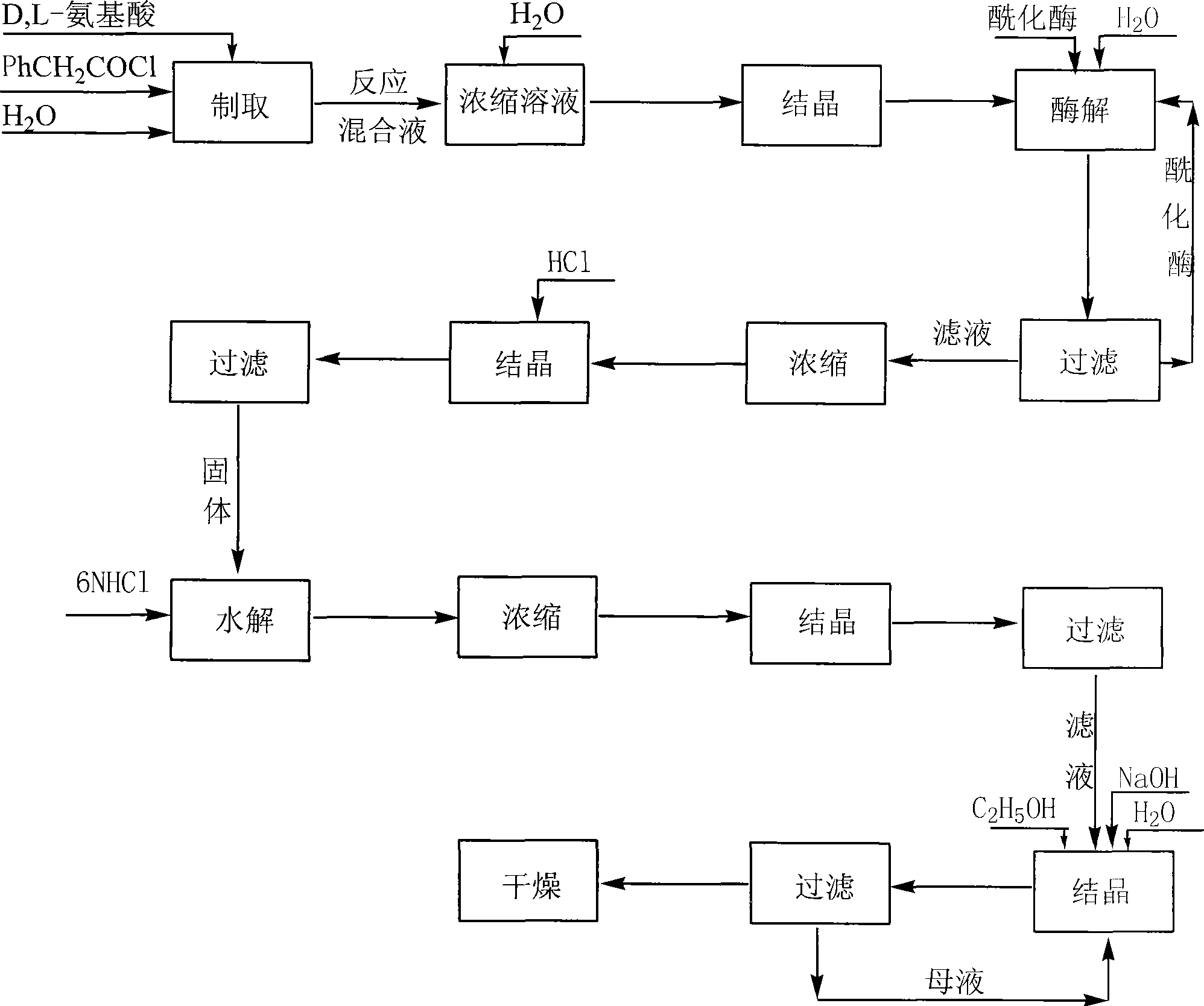
[0019] Embodiment 1: the production technology of D-methionine
[0020] 1. Synthesis of N-phenylacetyl-DL-methionine:
[0021] Add 298 grams of DL-methionine, 240 grams of NaOH, and 4,000 milliliters of water into a 10-liter reactor, stir well at 10°C, and add 387.5 grams of phenylacetyl chloride dropwise while stirring. During the reaction, control the pH=8-10, and the temperature Within 10°C, after the dropwise addition, keep it warm for 2 hours, then continue to stir the reaction at room temperature for 5 hours, stop the reaction, add concentrated hydrochloric acid to adjust the pH to 1-2, a large amount of white solid precipitates, vacuum filter to obtain a white solid. The filtrate was concentrated under reduced pressure (vacuum degree 0.1 p.m, temperature 60° C.) to make it 1 / 20 (240 ml) of the original volume, cooled, filtered the precipitated white solid, combined the white solid obtained twice, and put it into the next step reaction.
[0022] 2. Enzymatic hydrolysis ...
Embodiment 2
[0029] Embodiment 2: the production technology of D-alanine
[0030] 1. Synthesis of N-phenylacetyl-DL-alanine:
[0031]Add 365 grams of DL-alanine, 340 grams of NaOH, and 2,000 milliliters of water into a 5-liter reactor, stir fully at 10°C, and add 697.5 grams of phenylacetyl chloride dropwise while stirring, and control pH=8-10 during the reaction , the temperature is within 10°C, after the dropwise addition is completed, keep it warm for 2 hours, then continue to stir the reaction at room temperature for 5 hours, stop the reaction, add concentrated hydrochloric acid to adjust the pH to 1-2, a large amount of white solid is precipitated, vacuum filtration, and white solid. The filtrate was concentrated under reduced pressure (vacuum degree 0.1 p.m, temperature 60° C.) to make it 1 / 20 (100 ml) of the original volume, cooled, filtered the precipitated white solid, combined the white solid obtained twice, and put it into the next reaction.
[0032] 2. Enzymatic hydrolysis of...
Embodiment 3
[0039] Embodiment 3: the production technology of D-glutamic acid
[0040] 1. Synthesis of N-phenylacetyl-DL-glutamic acid:
[0041] Add 500 grams of DL-glutamic acid, 460 grams of NaOH, and 3,000 milliliters of water into a 5-liter reactor, stir well at 10°C, add 573.5 grams of phenylacetyl chloride dropwise under stirring, and control pH=8-10 during the reaction , the temperature is within 10°C, after the dropwise addition is completed, keep it warm for 2 hours, then continue to stir the reaction at room temperature for 5 hours, stop the reaction, add concentrated hydrochloric acid to adjust the pH to 1-2, a large amount of white solid is precipitated, vacuum filtration, and white solid. The filtrate was concentrated under reduced pressure (vacuum degree 0.1p.m, temperature 60°C) to make it 1 / 20 (150 ml) of the original volume, cooled, filtered the precipitated white solid, combined the white solid obtained twice, and put it into the next reaction.
[0042] 2. Enzymatic hy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com