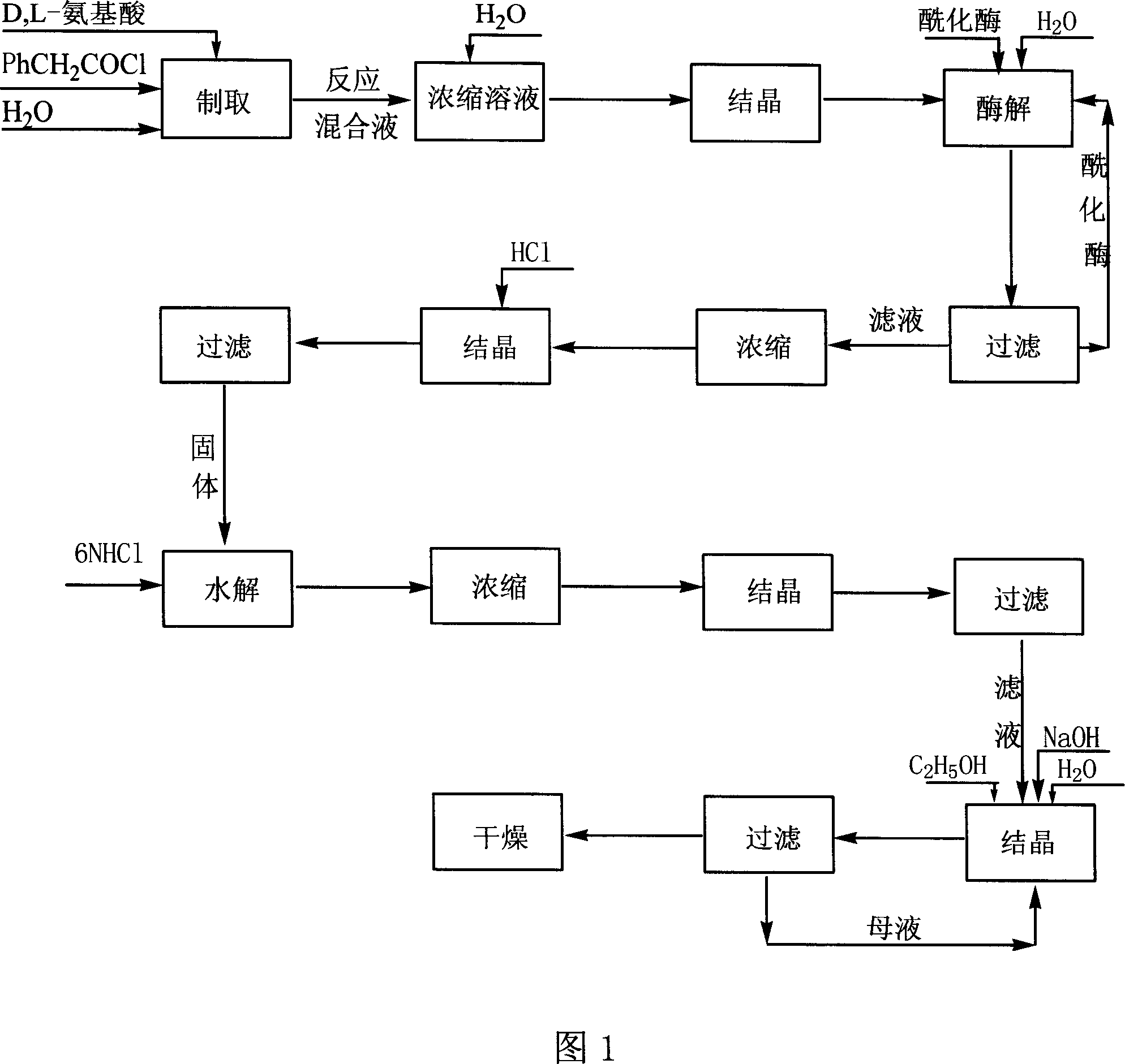
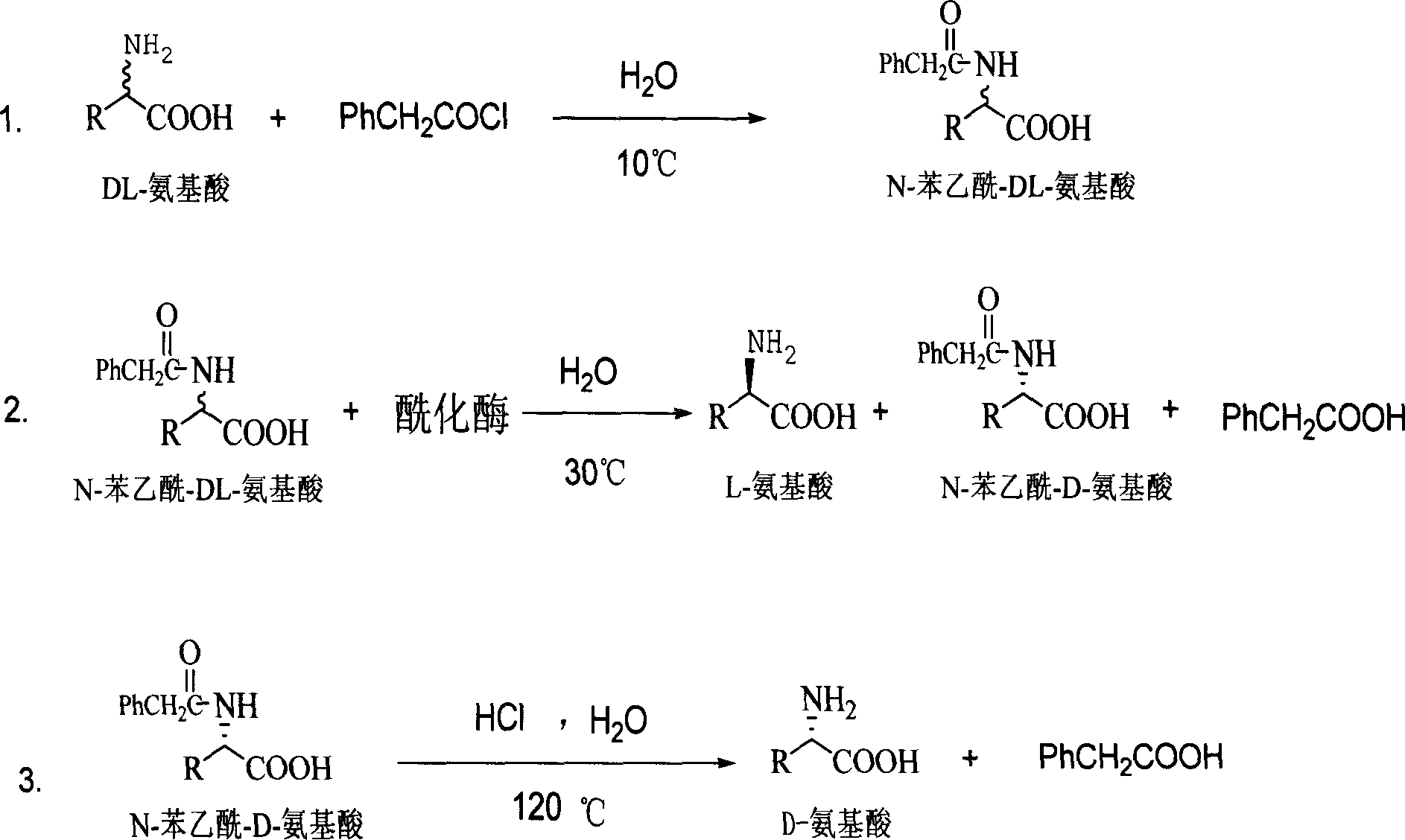
Method for producing D amino acid by immobilizing acylation enzyme of penicillin
A technology of amino acid and methionine, which is applied in the field of D-amino acid production technology, can solve the problems of high production cost and limitation of large-scale application of hydantoin, and achieve the effects of low production cost, short split route and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment one: the production technology of D-methionine
[0025] 1. Synthesis of N-phenylacetyl-DL-methionine:
[0026] Add 298 grams of DL-methionine, 240 grams of NaOH, and 4,000 milliliters of water into a 10-liter reactor, stir well at 10°C, and add 387.5 grams of phenylacetyl chloride dropwise while stirring. During the reaction, control the pH=8-10, and the temperature Within 10°C, after the dropwise addition, keep it warm for 2 hours, then continue to stir the reaction at room temperature for 5 hours, stop the reaction, add concentrated hydrochloric acid to adjust the pH to 1-2, a large amount of white solid precipitates, vacuum filter to obtain a white solid. The filtrate was concentrated under reduced pressure (vacuum degree 0.1 p.m, temperature 60° C.) to make it 1 / 20 (240 ml) of the original volume, cooled, filtered the precipitated white solid, combined the white solid obtained twice, and put it into the next step reaction.
[0027] 2. Enzymatic hydrolysi...
Embodiment 2
[0034] Embodiment two: the production technology of D-alanine
[0035] 1. Synthesis of N-phenylacetyl-DL-alanine:
[0036]Add 365 grams of DL-alanine, 340 grams of NaOH, and 2000 milliliters of water into a 5-liter reactor, and stir thoroughly at 10°C.
[0037] Add 697.5 grams of phenylacetyl chloride dropwise. During the reaction, control the pH to 8-10 and keep the temperature within 10°C. After the dropwise addition, keep the temperature for 2 hours, then continue to stir and react at room temperature for 5 hours, stop the reaction, and add concentrated hydrochloric acid to lower the pH. Adjusted to 1-2, a large amount of white solid precipitated out, and was vacuum filtered to obtain a white solid. The filtrate was concentrated under reduced pressure (vacuum degree 0.1 p.m, temperature 60° C.) to make it 1 / 20 (100 ml) of the original volume, cooled, filtered the precipitated white solid, combined the white solid obtained twice, and put it into the next reaction.
[0038]...
Embodiment 3
[0045] Embodiment three: the production technology of D-glutamic acid
[0046] 1. Synthesis of N-phenylacetyl-DL-glutamic acid:
[0047] Add 500 grams of DL-glutamic acid, 460 grams of NaOH, and 3,000 milliliters of water into a 5-liter reactor, stir well at 10°C, add 573.5 grams of phenylacetyl chloride dropwise under stirring, and control pH=8-10 during the reaction , the temperature is within 10°C, after the dropwise addition is completed, keep it warm for 2 hours, then continue to stir the reaction at room temperature for 5 hours, stop the reaction, add concentrated hydrochloric acid to adjust the pH to 1-2, a large amount of white solid is precipitated, vacuum filtration, and white solid. The filtrate was concentrated under reduced pressure (vacuum degree 0.1p.m, temperature 60°C) to make it 1 / 20 (150 ml) of the original volume, cooled, filtered the precipitated white solid, combined the white solid obtained twice, and put it into the next reaction.
[0048] 2. Enzymati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com