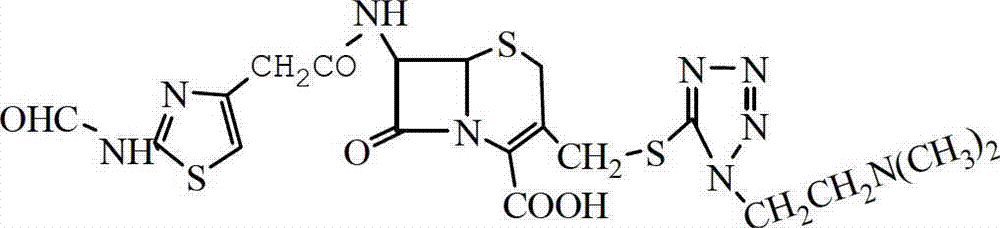
Preparation method of cefotiam hydrochloride
A technology of cefotiam hydrochloride and base thiazole acetic acid, which is applied in the field of antibiotic drug synthesis and achieves the effects of high purity and yield, mild reaction conditions and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
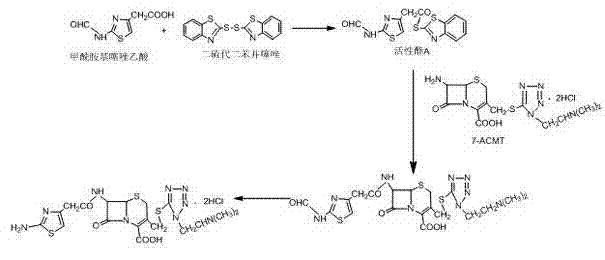
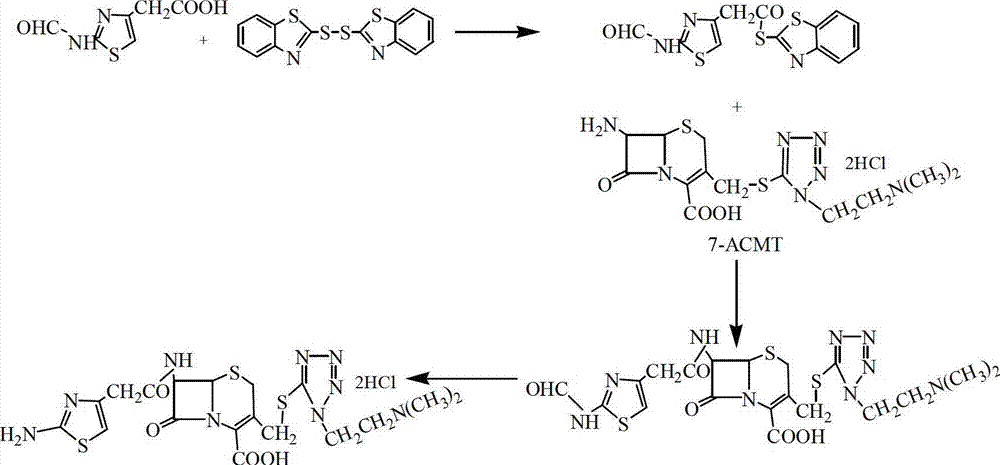
[0028] Add 30mL acetone, 20mL CH 2 Cl 2 , stirred at 15°C for 10 min, added 11 g of formamidothiazole acetic acid, stirred and added 4.5 mL of triethylamine, stirred for 15 min, added 22 g of dithiodibenzothiazole at room temperature, stirred for 10 min, slowly added 20 mL of Triethyl phosphate was kept at 28°C for 1 hour, cooled to 0°C, suction filtered, washed, and dried to obtain 17.6g of active ester A, the content of which was detected by high performance liquid chromatography was 99.5%.
[0029] Add 20g ACMT and 140mL dichloromethane into a 500mL three-necked bottle, stir and add 15.2g active ester A, cool down to 8°C, add 5mL ethanol, add 20mL triethylamine dropwise, time the reaction for 3.5h after the dropwise addition, and add Add 5mL of ethanol twice, (the ADMT residual is 0.48% as detected by HPLC), add 45mL of aqueous solution (containing 0.5g of EDTA, 0.5g of sodium bisulfite) and let stand, and take the water layer. Add 8mL of concentrated hydrochloric acid to...
Embodiment 2
[0031] Add 40mL acetone, 20mL CH 2 Cl 2 , Stir at 15°C for 15 min, add 11 g of formamidothiazole acetic acid, stir and add triethylamine, stir for 15 min, add 30 g of dithiodibenzothiazole at room temperature, slowly add 25 mL of triethyl phosphite dropwise with an acid burette, Keep warm at 5°C for 3 hours, cool down to 0°C, filter with suction, wash the material, and dry to obtain 17.2g of active ester A, the content detected by high performance liquid chromatography is 99.2%.
[0032] Add 20g ACMT and 140mL dichloroethane into a 500mL three-necked bottle, stir and add 18g active ester A, cool down to 15°C, add 5mL ethanol, drop 16mL triethylamine, and react for 2 hours after the dropwise addition, adding 5mL every 30min Ethanol was added twice, (the residual ACMT detected by HPLC was 0.5%), 45mL of aqueous solution (containing 0.5g of EDTA, 0.5g of sodium hydrosulfite) was added and allowed to stand, and the water layer was taken. Add 9mL of concentrated hydrochloric acid...
Embodiment 3
[0034] Add 20mL acetone, 20mL CH 2 Cl 2 , stirred at 15°C for 10 min, added 11 g of formamidothiazole acetic acid, stirred and added 0.5 g of sodium bicarbonate, stirred for 15 min, added 20 g of dithiodibenzothiazole at room temperature, stirred for 10 min, slowly added dropwise 30 mL of sodium bicarbonate with an acid burette Triethyl phosphate was kept at 40°C for 1.5 hours, cooled to 0°C, suction filtered, washed, and dried to obtain 17.9g of active ester A, the content of which was detected by high performance liquid chromatography was 99.0%.
[0035] Add 20g ACMT and 140mL tetrahydrofuran into a 500mL three-necked bottle, stir and add 22g of active ester A, cool down to 20°C, add 5mL of ethanol, dropwise add 24mL of 15% ammonia water, and react for 4 hours after the dropwise addition, and add 5mL of ethanol every 30min for a total of Twice, (the residual ACMT detected by HPLC is 0.5%), add 45mL of aqueous solution (containing 0.5g of EDTA, 0.5g of sodium bisulfite) and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com