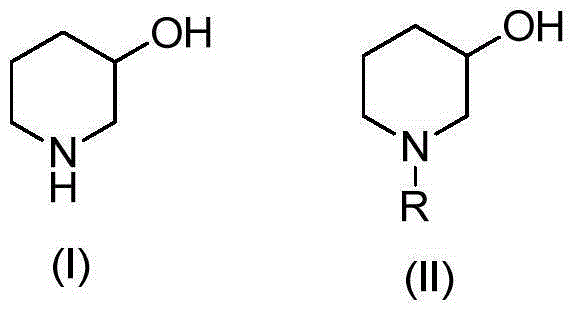
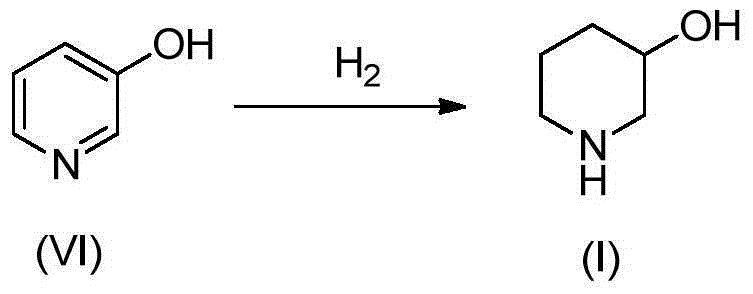
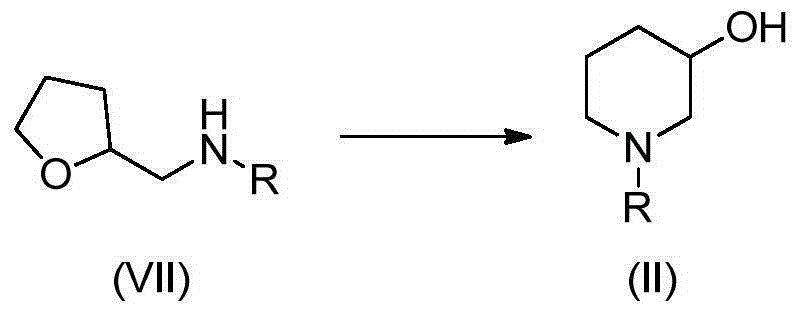
Preparation method of 3-hydroxypiperidine, preparation method of derivative of 3-hydroxypiperidine, and intermediate of 3-hydroxypiperidine
A technology of hydroxypiperidine and hydroxypentylamine hydrohalide, which is applied in the field of preparation of 3-hydroxypiperidine and its derivatives and its intermediates, and can solve the problems of unavailable raw materials, harsh reaction conditions, and low production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] (1) Preparation of 5-chloro-2-hydroxypentylamine hydrochloride ((1-2)
[0055]
[0056] Add 100mL of concentrated hydrochloric acid dropwise to 150.0g of tetrahydrofurfurylamine (1-1), control the rate of addition so that the reaction temperature is lower than 35°C, then continue to feed hydrogen chloride gas under stirring, slowly raise the temperature, and finally the reaction temperature reaches 80°C °C, react for 10 hours. The introduction of hydrogen chloride gas was stopped, and after cooling to room temperature, the reaction solution was concentrated under reduced pressure to remove most of the water, and a large amount of white solids were precipitated. Desalting was carried out by filtration, washed with cold water, and the resulting mother liquor was further concentrated to dryness to obtain 224.0 g of white solid 5-chloro-2-hydroxypentylamine hydrochloride (1-2), with a purity greater than 95% and a yield of 87%. 1 H NMR (400MHz,D 2 O)δ3.77(ddd, J=12.7,8...
Embodiment 2
[0064] (1) Preparation of 5-bromo-2-hydroxypentylamine hydrobromide ((2-2)
[0065]
[0066] Add 40mL of concentrated hydrobromic acid dropwise to 60.0g of tetrahydrofurfurylamine (1-1), control the rate of addition so that the reaction temperature is lower than 30°C, then continue to feed hydrogen bromide gas under stirring, slowly raise the temperature, and finally The reaction temperature reached 80°C, and the reaction was carried out for 8 hours. The introduction of hydrogen bromide gas was stopped, and after cooling to room temperature, the reaction solution was concentrated under reduced pressure to remove most of the water, and a large amount of white solids were precipitated. Desalting by filtration, washing with cold water, and further concentrating the resulting mother liquor to dryness yielded 86.0 g of 5-bromo-2-hydroxypentylamine hydrobromide (2-1), with a purity greater than 90% and a yield of 83%. 1 H NMR (400MHz,D 2 O)δ3.82(m,1H),3.57(t,J=6.6Hz,2H),3.06(dd...
Embodiment 3
[0074] Preparation of N-benzyloxycarbonyl-3-hydroxypiperidine (3-1)
[0075]
[0076] Dissolve 20.0g of 5-chloro-2-hydroxypentylamine hydrochloride ((1-2) in 50mL of water, add dropwise a solution of 20.4g of sodium hydroxide in 20mL of water at 10-15°C, and drop it in about 1 hour After dropping, react at about 15°C for 2 hours. Then heat up to 40-50°C and react for 4 hours. Cool to room temperature after stopping the reaction, add 80mL of dichloromethane and 20mL of water containing 7.5g of sodium hydroxide solution, and then Add 20.0g benzyl chloroformate, naturally rise to room temperature and react overnight. TLC spot plate, reaction is complete, separate liquid, and aqueous phase is extracted once with 50mL dichloromethane. Merge organic phase, organic phase is washed with water successively, and saturated saline is washed, without Drying over sodium sulfate over water, suction filtration, concentrated to obtain 25.0g colorless oily matter.The crude product was recrys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com