7Beta-methyl-tetralone derivative and preparation method and application thereof
A technology of methoxyphenyl and trifluoromethylphenyl, which is applied in the field of chemical pharmaceuticals and can solve problems such as analgesia, sedation, and respiratory depression addiction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
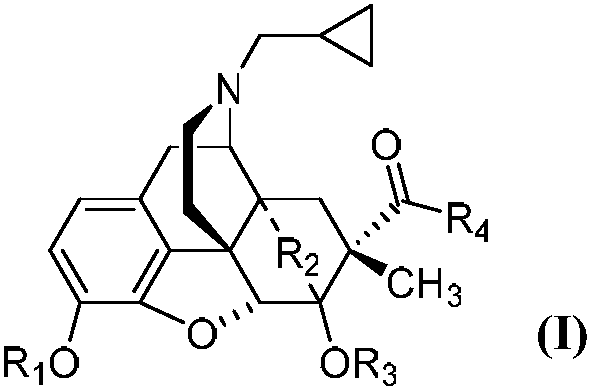
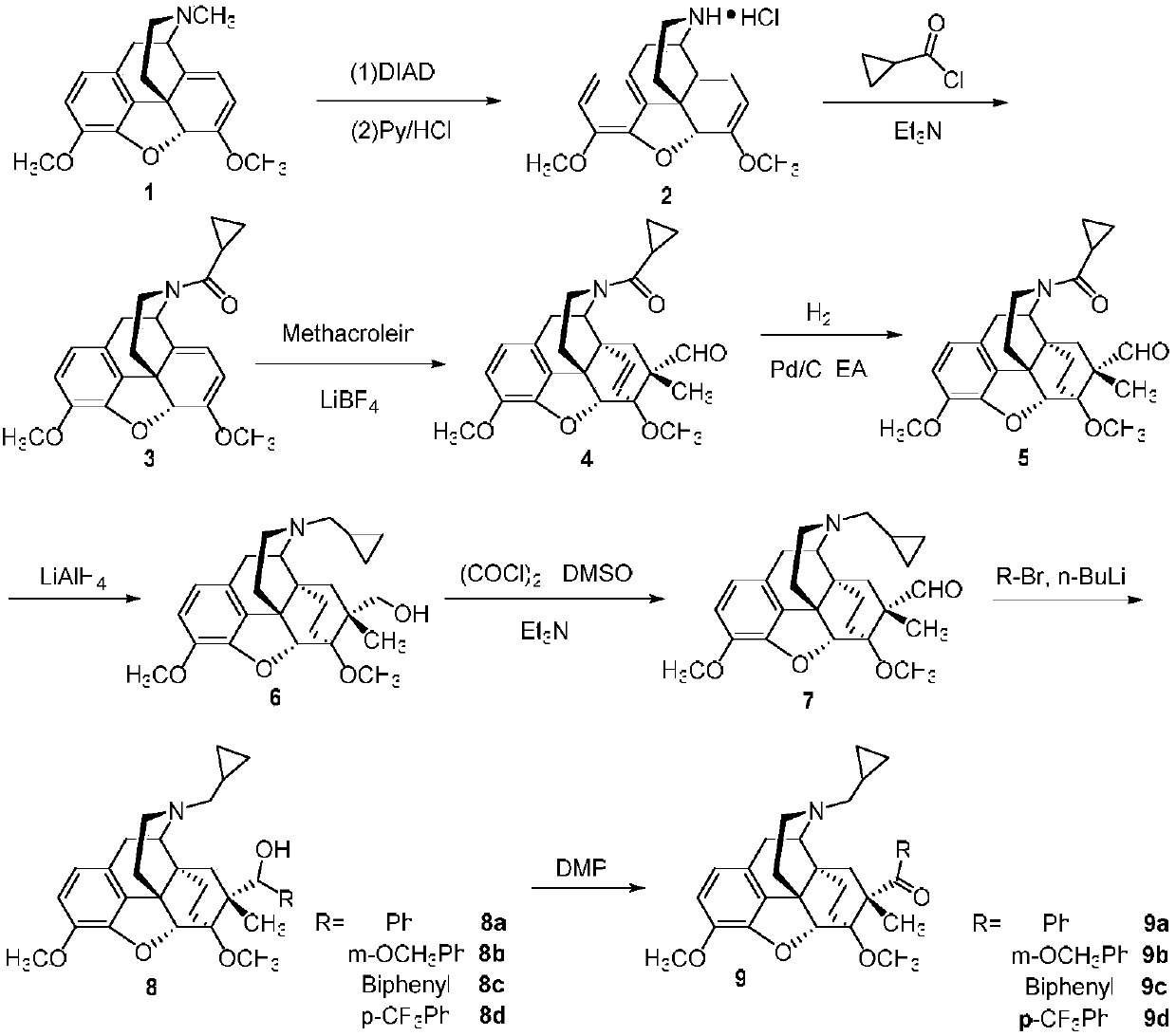
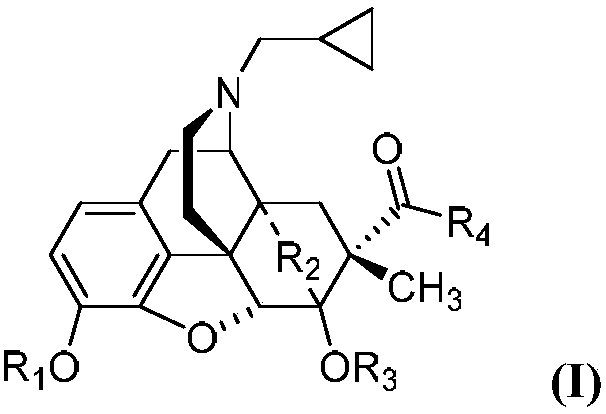
[0026] Embodiment 1 can prepare the compound with general formula (I) by the same method, wherein,
[0027] 2 preparation
[0028] Under the protection of argon, add thebaine (500mg, 1.6mmol, 1eq) anhydrous acetonitrile 5mL and diisopropyl azodicarboxylate (0.34mL, 1.7mmol, 1.1eq) into the three-necked flask, and reflux for 4h , cooled to room temperature overnight. The reaction solution was spin-dried, the residue was dissolved in 5 mL of methanol, and pyridine hydrochloric acid (185 mg, 1.6 mmol, 1 eq) was added and stirred for two days. Suction filtration, wash the filter cake with methanol and ethyl acetate to obtain 215mg of white solid 1, yield 40%, melting point: 260.3°C (decomposition point);
[0029] 1H-NMR (400MHz, CDCl3) δ6.68(d, J=8.2Hz, 1H), 6.62(d, J=8.2Hz, 1H), 5.50(d, J=6.4Hz, 1H), 5.27(s, 1H), 5.03(d, J=6.4Hz, 1H), 3.85(s, 3H), 3.60(s, 3H), 3.17(ddd, Ja=17.6, Jb=13.8, Jc=4.2Hz, 3H), 2.93 (dd, Ja=13.7Hz, Jb=4.8 Hz, 1H), 2.07(td, Ja=12.5Hz, Jb=5.1Hz, 3H), 1....
Embodiment 2
[0067] Example 2 Radioactive Ligand Binding Experiment
[0068]The compounds used in the pharmacological binding screening were all in free base form. The experiment is divided into total binding tubes and non-specific binding tubes, and several sets of sample tubes are set up to add the compounds to be screened. Add 80uL of cell suspension expressing opioid κ, μ and δ receptors to the total binding tube, add [3H]U69593, [3H]DAMGO, [3H]DPDPE respectively to the three tubes (final concentration is 0.35nM) 10uL; Add U50488, DAMGO, and DPDPE to the corresponding non-specific tubes to make the final concentration 1uM; add 10ul of the same concentration of drugs to the sample tube (final concentration is 1uM), and adjust to a final volume of 100uL with 50mM Tris.HCl (pH 7.4). Incubate at 37°C for 30 min, then place in an ice bath to terminate the reaction. Suction filtration through GF / C (Whatman) glass fiber filter paper on the Millipore sample collector. Rinse three times with...
Embodiment 335
[0072] Example 3[ 35 S] GTPγS binding assay
[0073] Measuring protein concentration with Bradford Protein Concentration Assay Kit: Add standard protein BSA at concentrations of 0, 50, 100, 200, 250 μg / ml and samples to be tested to 96-well plates, add 20 μl to each well; add G250 to each well 200 μl of staining solution was placed at room temperature for 3-5 minutes, and the absorbance at A595 wavelength was measured with a microplate reader. The protein concentration was calculated from the standard curve.
[0074] Table 2
[0075]
[0076] The prepared membrane receptors were diluted with reaction buffer (R.B) to the desired concentration, and samples were added as shown in Table 2 (unit: μl). The reaction tube was incubated in a water bath at 27°C for 1 hour, filtered under reduced pressure with a glass fiber membrane and counted by liquid scintillation. Calculate according to the following formula:
[0077] [ 35 S] GTPγS binding rate=100×(cpm sample -cpm non-sp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com