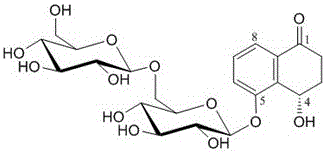
Compound 4(S)-4,5-dihydroxy-alpha-tetralone 5-O-beta-D-glucopyranose (1->6)-beta-D-glucopyranoside, and preparation method and application thereof
A technology of glucopyranoside and glucopyranose, which is applied to the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of wasting medicinal resources and polluting the environment, and achieve the prevention of shortages and the rational use of resources , expanding the effect of drug sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0032] Compound Identification:
Embodiment 1
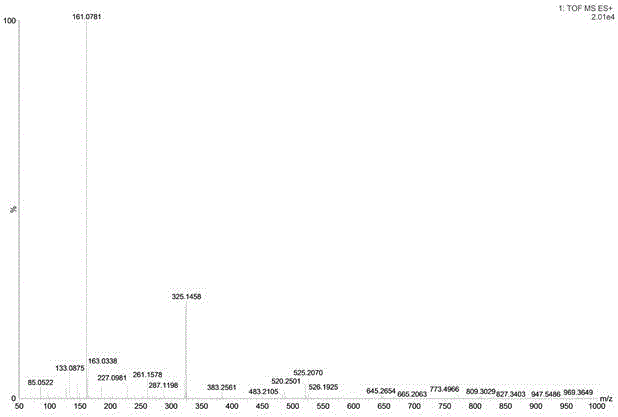
[0033] The compound obtained in Example 1 is a yellow amorphous powder (MeOH). UV spectrum (MeOH) exhibits maximum absorption at 254 nm. Positive HR-ESI-MS spectra, such as figure 2 As shown, [M+Na] can be seen at m / z525.1657 + Ion peak, indicating that the molecular weight of the compound is 502. combine 1 H-NMR, 13 C-NMR and DEPT spectra speculate that its molecular formula is C 22 h 30 o 13 , calculate its degree of unsaturation to be 8.
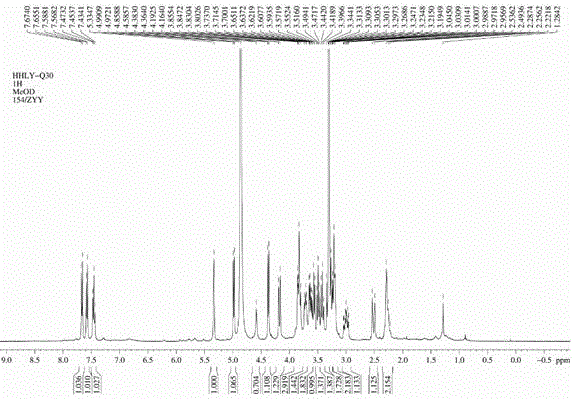
[0034] in the compound 1 H-NMR (CD 3 OD,400MHz) spectrum, such as image 3 As shown, the low field area δ7.58 (1H, dd, J=8.0,0.9Hz), 7.45 (1H, t, J=8.0Hz) and 7.66 (1H, dd, J=8.0,0.9Hz) is a group Aromatic proton signals of the ABX coupled system. In the high field region δ2.26 (2H, m), 3.00 (1H, ddd, J=17.0, 12.4, 5.6Hz) and 2.51 (1H, brd, J=17.0Hz) are two methylenes on the naphthalene ring Primary proton signal. At δ5.33brs is a methine proton signal. At δ4.98 (1H, d, J=7.5Hz) and 4.37 (1H, d, J=7.6Hz), there are two si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com