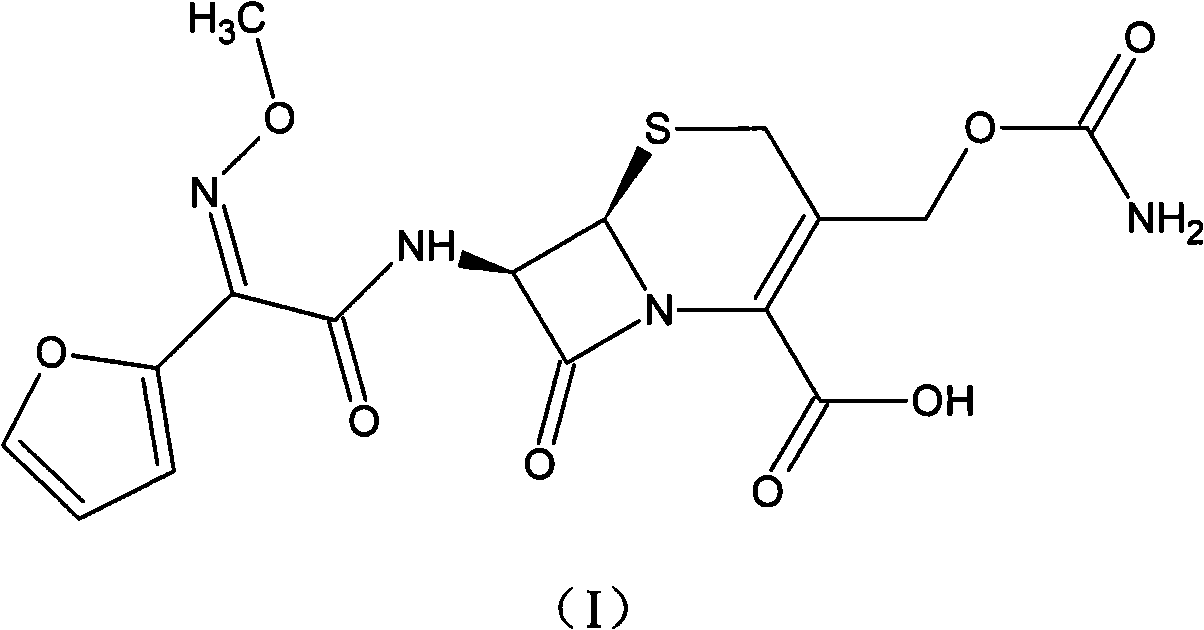
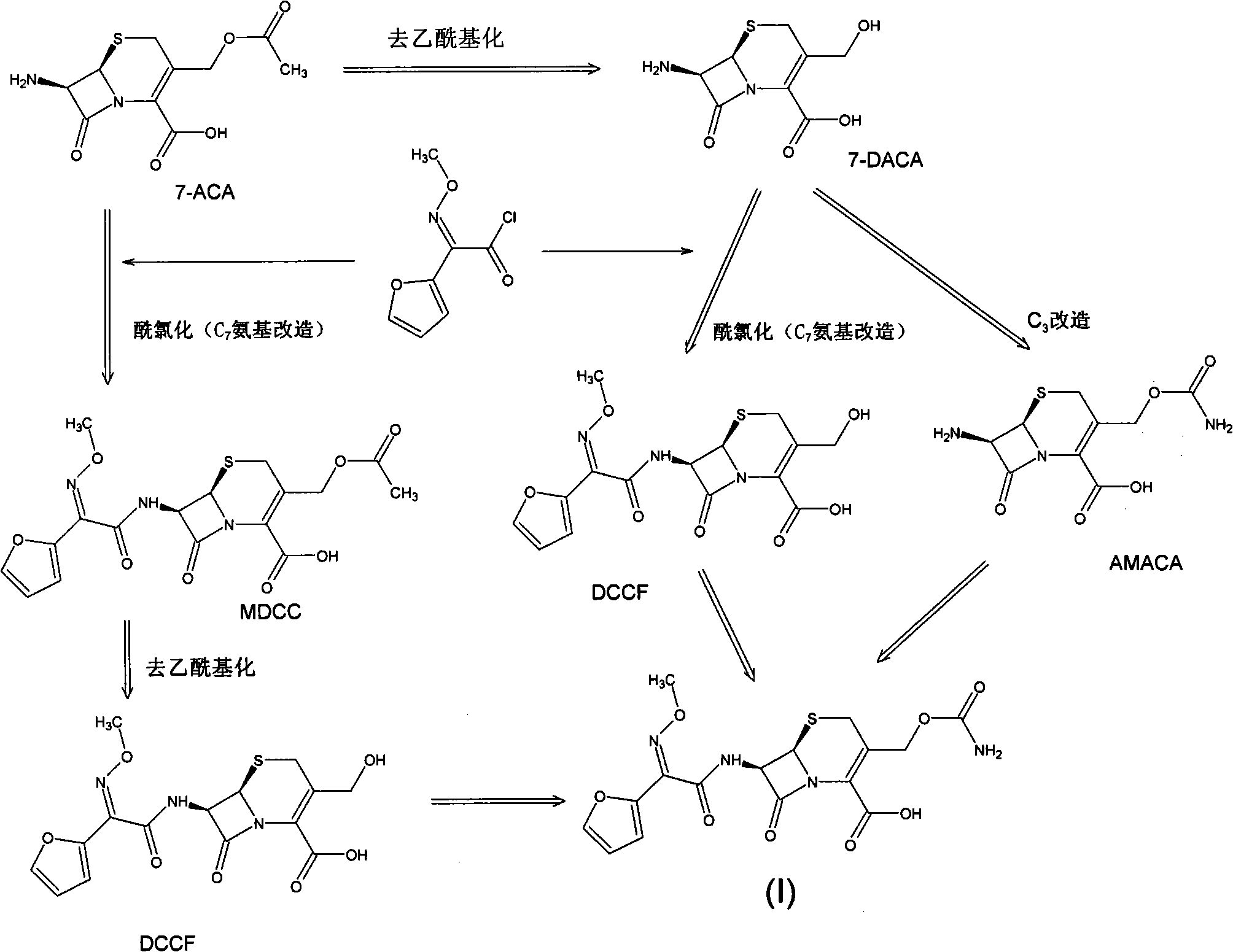
Method for preparing cefuroxime acid
A technology of cefuroxime acid and furoxine, which is applied in the new synthesis field of cefuroxime acid, can solve the problems of cefuroxime acid product discoloration, product purity decline, consumption, etc., and achieves avoiding organic solvent crystallization, less impurity content, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Add 50Kg of phosphorus pentachloride and 450Kg of dichloromethane into a dry reaction kettle and stir, cool to -20°C, add 70Kg of dimethylacetamide dropwise, and add sodium methoxyiminofuranacetate at -15°C 40Kg stirred and reacted for 90min. Add 170Kg of water at a constant temperature of -5°C and stir, then let stand to separate layers. After the organic phase of the lower layer was separated, 10% NaCl aqueous solution was added, stirred, and allowed to stand to separate layers. The organic phase was separated and frozen to -10°C until use.
[0039] (2) 230Kg of pure water, 50Kg of 7-ACA, 0.25Kg of EDTA, and 0.5kg of sodium metabisulfite were stirred at 0°C, and 15% aqueous sodium hydroxide solution was added dropwise to adjust the pH to 8, and stirred until the solid dissolved.
[0040] (3) Add the organic phase prepared in (1) dropwise to the solution prepared in (2), the temperature is controlled at 0° C., and the pH of the reaction solution is maintained at ...
Embodiment 2
[0045] (1) Add 50Kg of phosphorus pentachloride and 450Kg of dichloromethane into a dry reaction kettle and stir, cool to -20°C, add 70Kg of dimethylacetamide dropwise, and add sodium methoxyiminofuranacetate at -15°C 40Kg stirred and reacted for 90min. Add 170Kg of water at a constant temperature of -5°C and stir, then let stand to separate layers. After the organic phase of the lower layer was separated, 10% NaCl aqueous solution was added, stirred, and allowed to stand to separate layers. The organic phase was separated and frozen to -10°C until use.
[0046] (2) 230Kg of pure water, 50Kg of 7-ACA, 0.25Kg of EDTA, and 0.5kg of sodium metabisulfite were stirred at 0°C, and 15% aqueous sodium hydroxide solution was added dropwise to adjust the pH to 9, and stirred until the solid dissolved.
[0047] (3) The organic phase prepared in (1) was added dropwise to the solution prepared in (2), the temperature was controlled at -2°C, and the pH of the reaction solution was maintai...
Embodiment 3
[0052] (1) Add 50Kg of phosphorus pentachloride and 450Kg of dichloromethane into a dry reaction kettle and stir, cool to -20°C, add 70Kg of dimethylacetamide dropwise, and add sodium methoxyiminofuranacetate at -15°C 40Kg stirred and reacted for 90min. Add 170Kg of water at a constant temperature of -5°C and stir, then let stand to separate layers. After the organic phase of the lower layer was separated, 10% NaCl aqueous solution was added, stirred, and allowed to stand to separate layers. The organic phase was separated and frozen to -10°C until use.
[0053] (2) 230Kg pure water, 50Kg 7-ACA, 0.25Kg EDTA, 0.5kg sodium metabisulfite, stir at 0°C, add dropwise 15% sodium hydroxide aqueous solution, adjust pH=8, stir until the solid dissolves.
[0054] (3) Add the organic phase prepared in (1) dropwise to the solution prepared in (2), the temperature is controlled at 0° C., and the pH of the reaction solution is maintained at 6.5 to 7.0 by dropping 15% aqueous sodium hydroxi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com