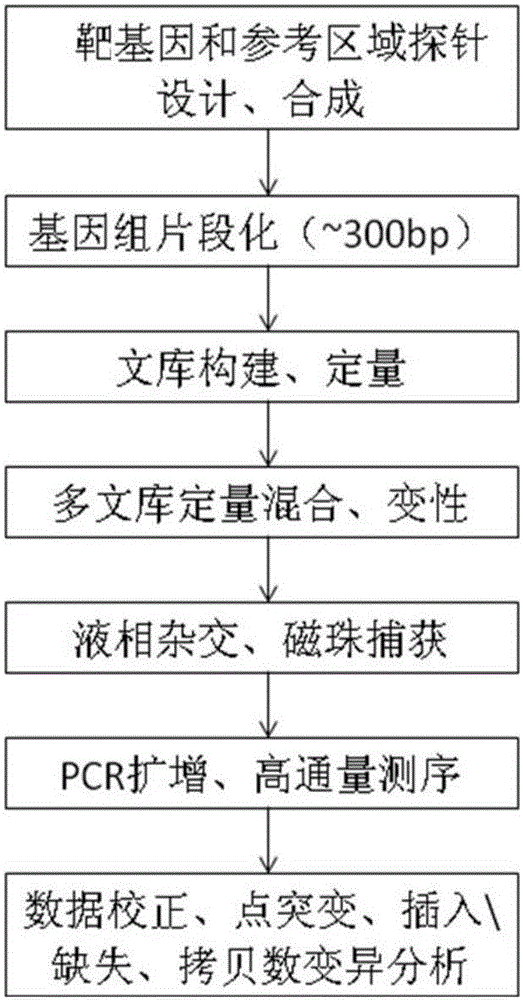
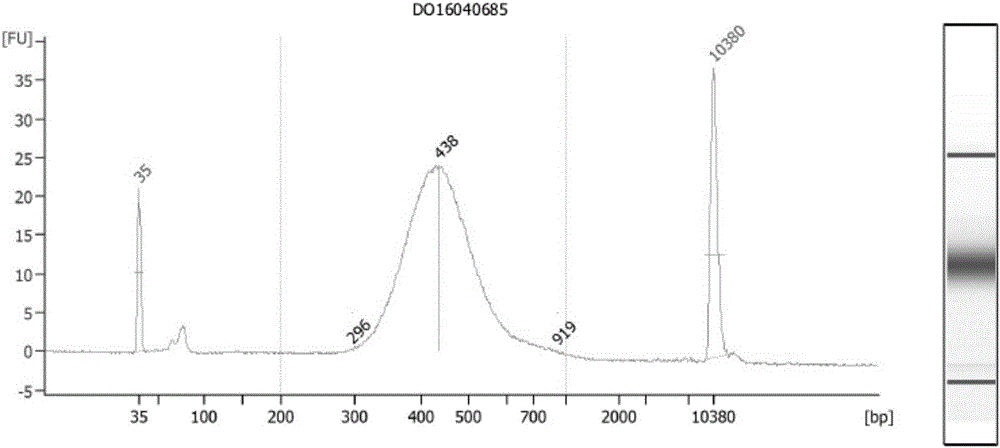
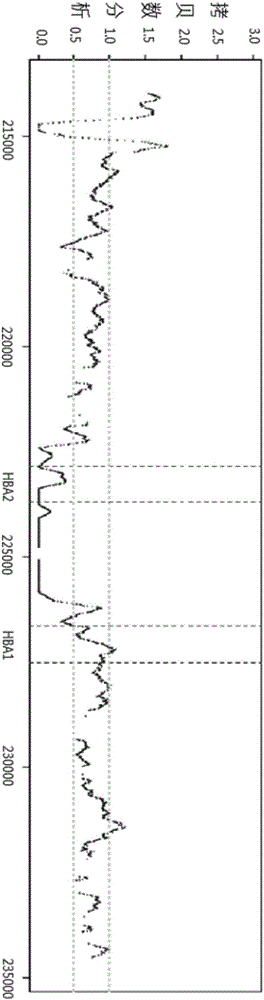
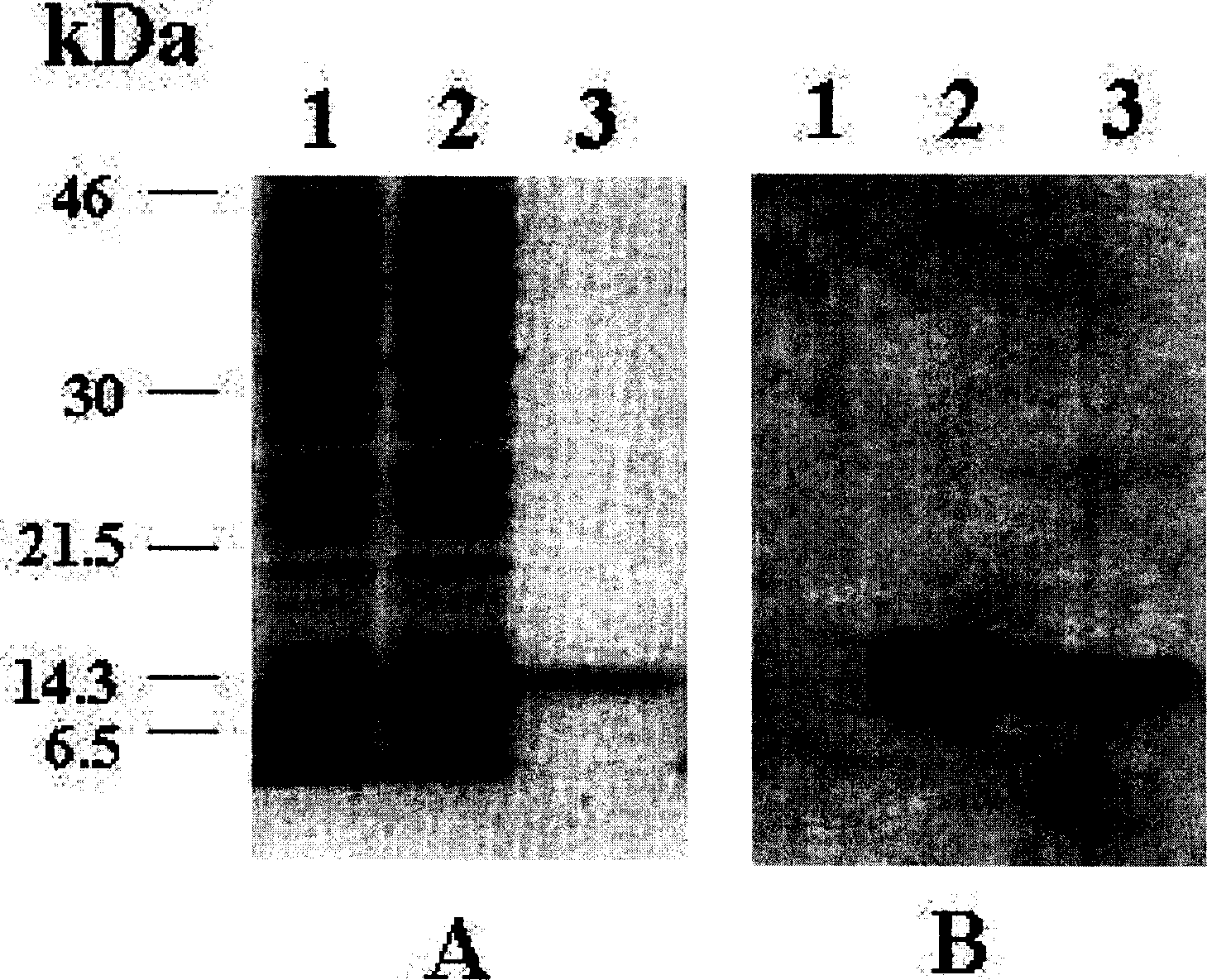
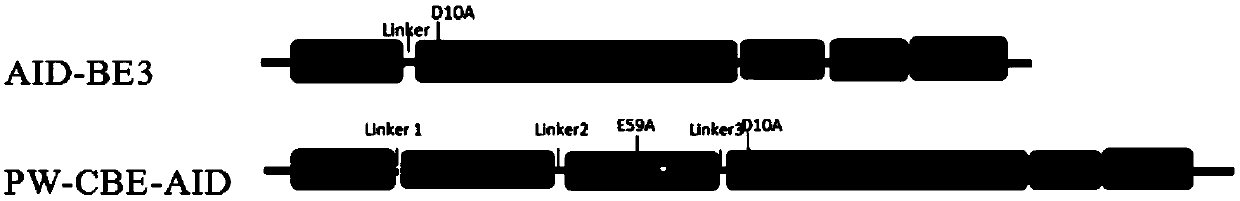
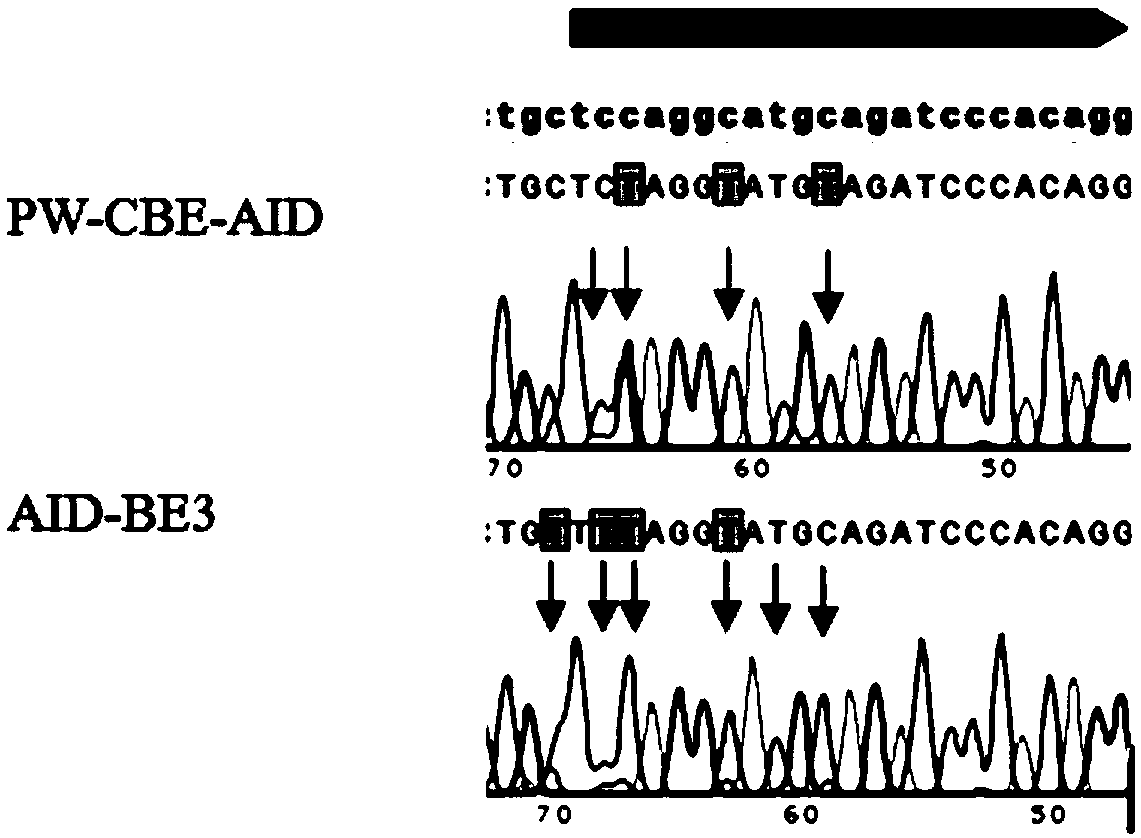
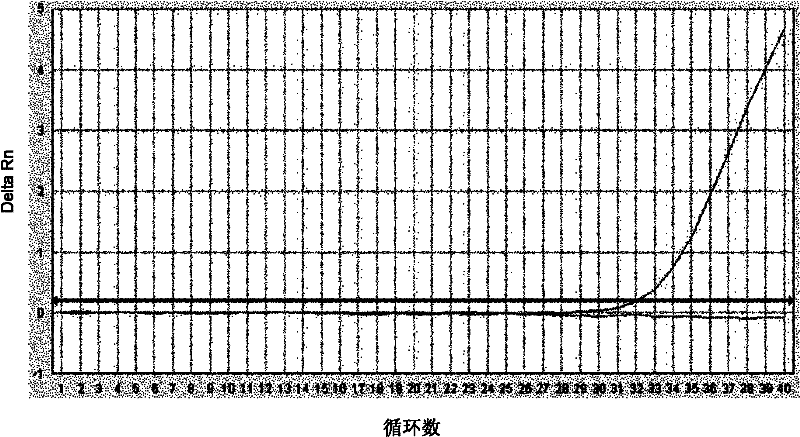
Patents
Literature
318 results about "Deletion mutation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Deletion (genetics) In genetics, a deletion (also called gene deletion, deficiency, or deletion mutation) (sign: Δ) is a mutation (a genetic aberration) in which a part of a chromosome or a sequence of DNA is lost during DNA replication.
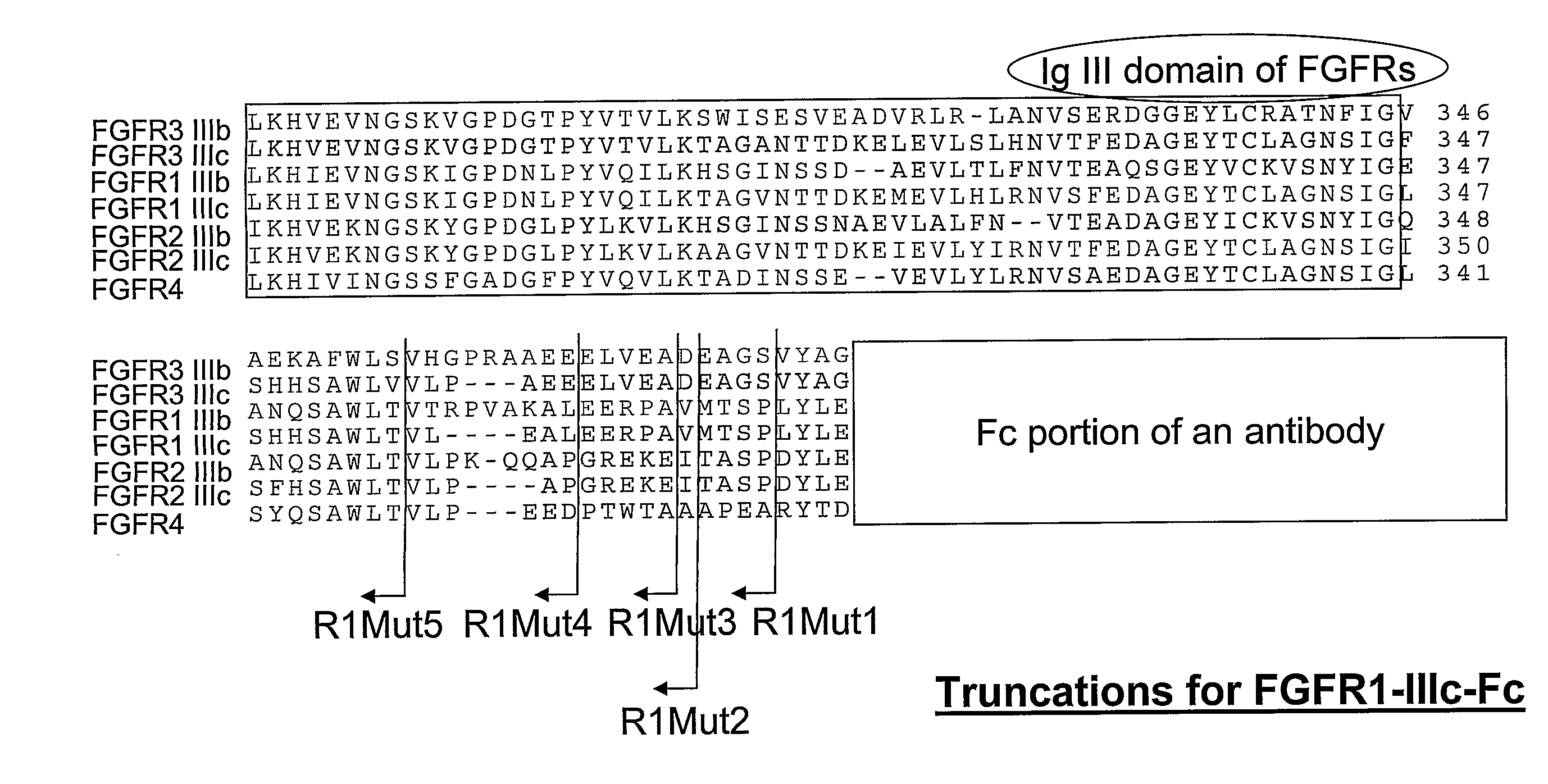
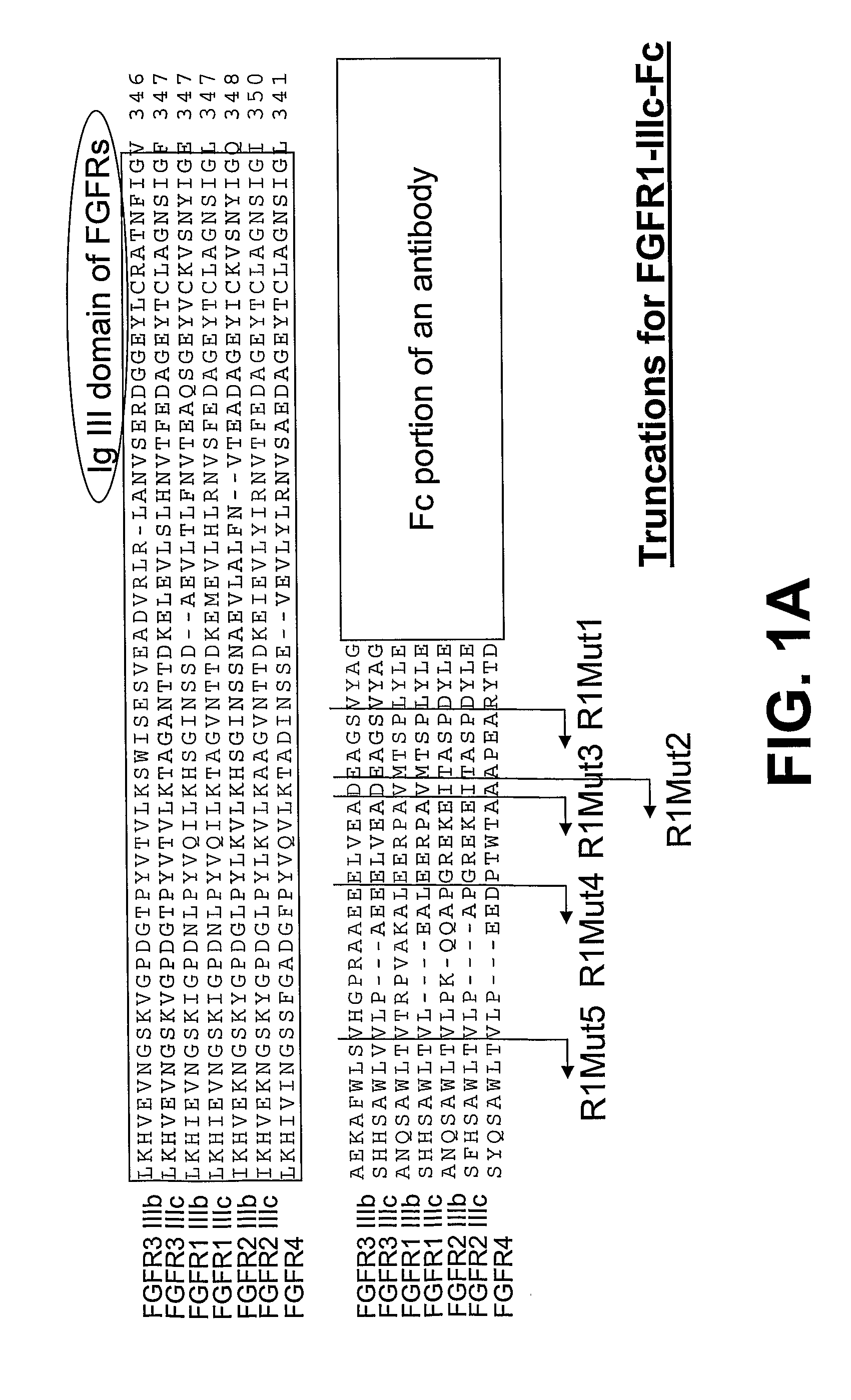
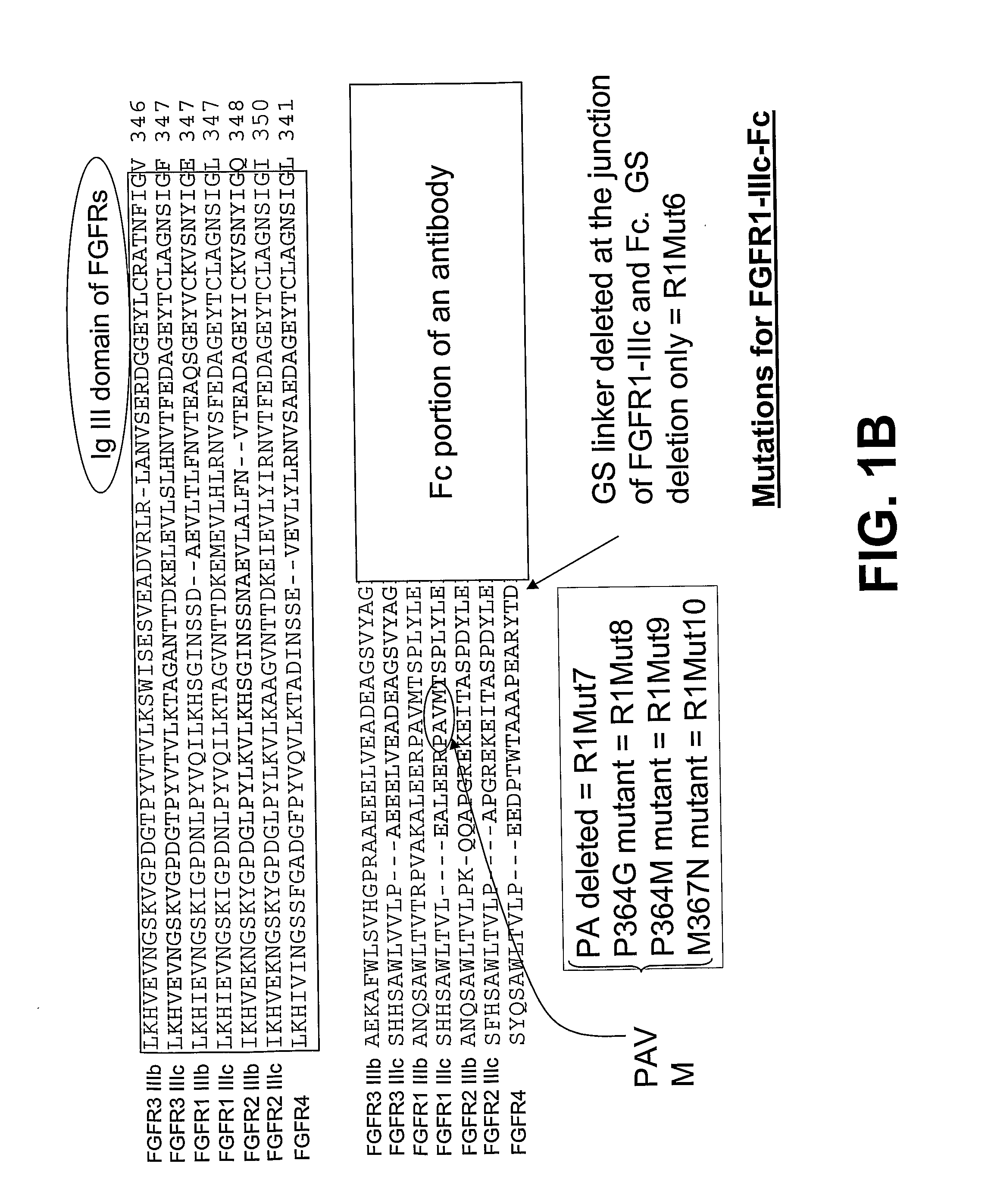
Compositions and methods of treating disease with FGFR fusion proteins
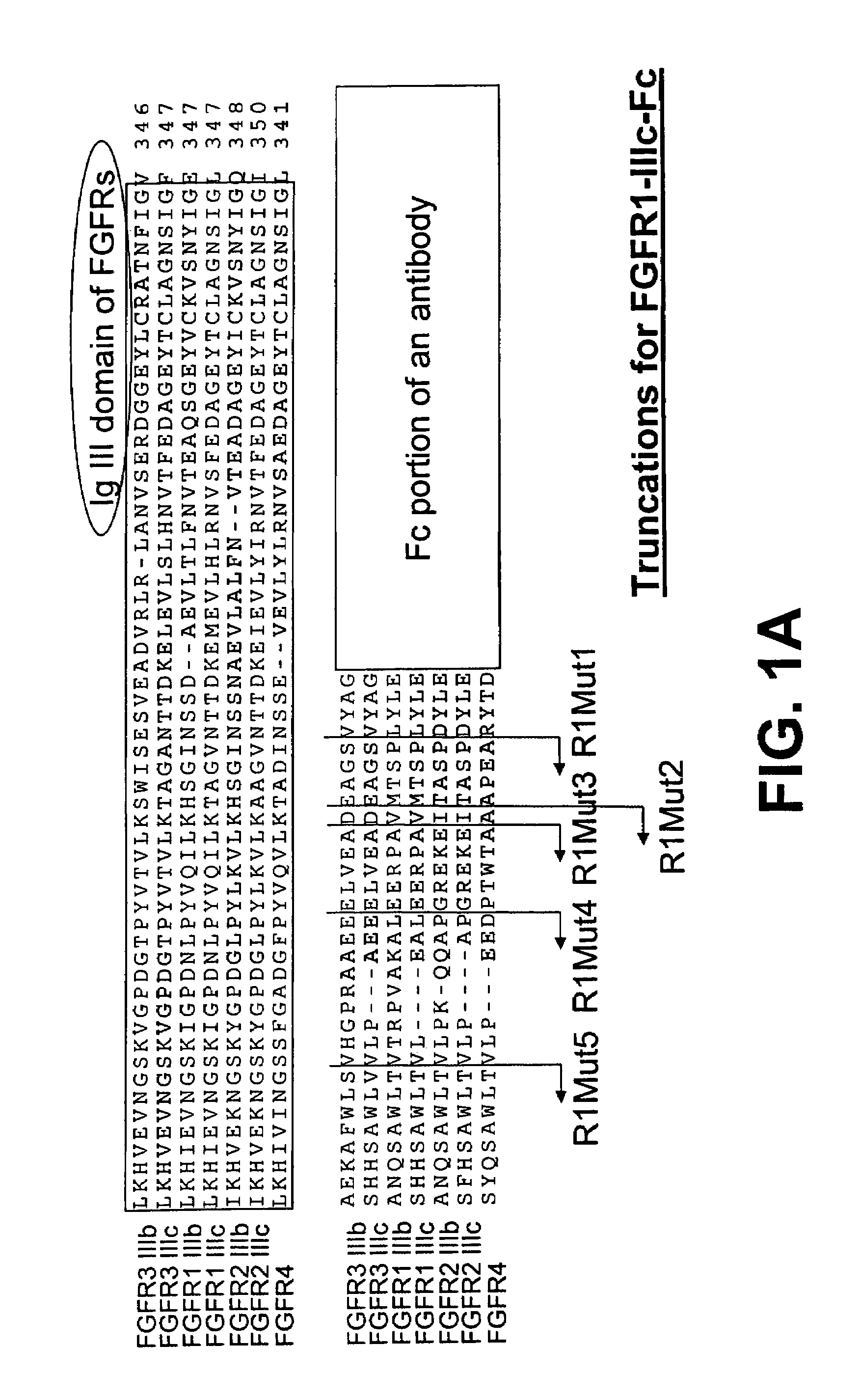
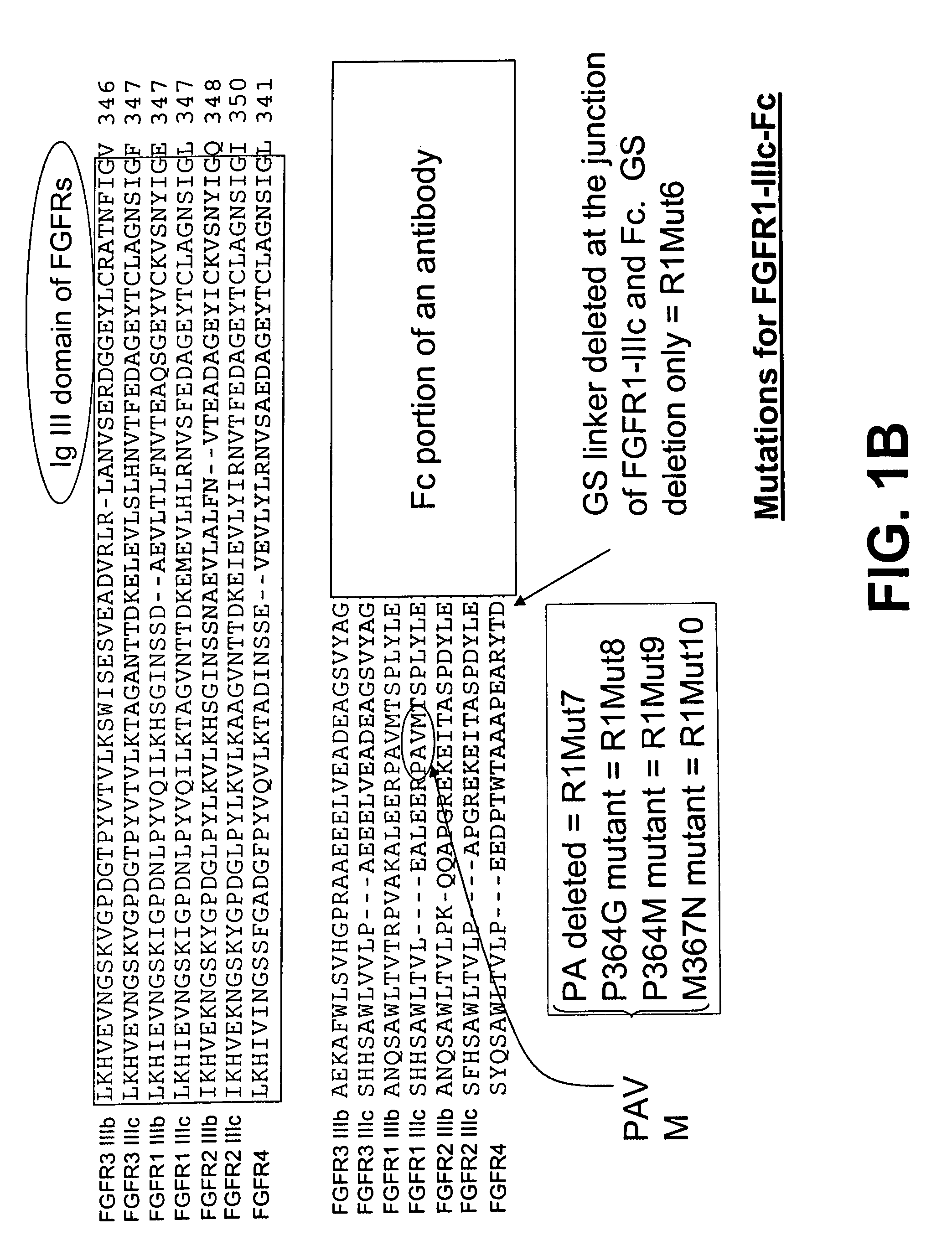
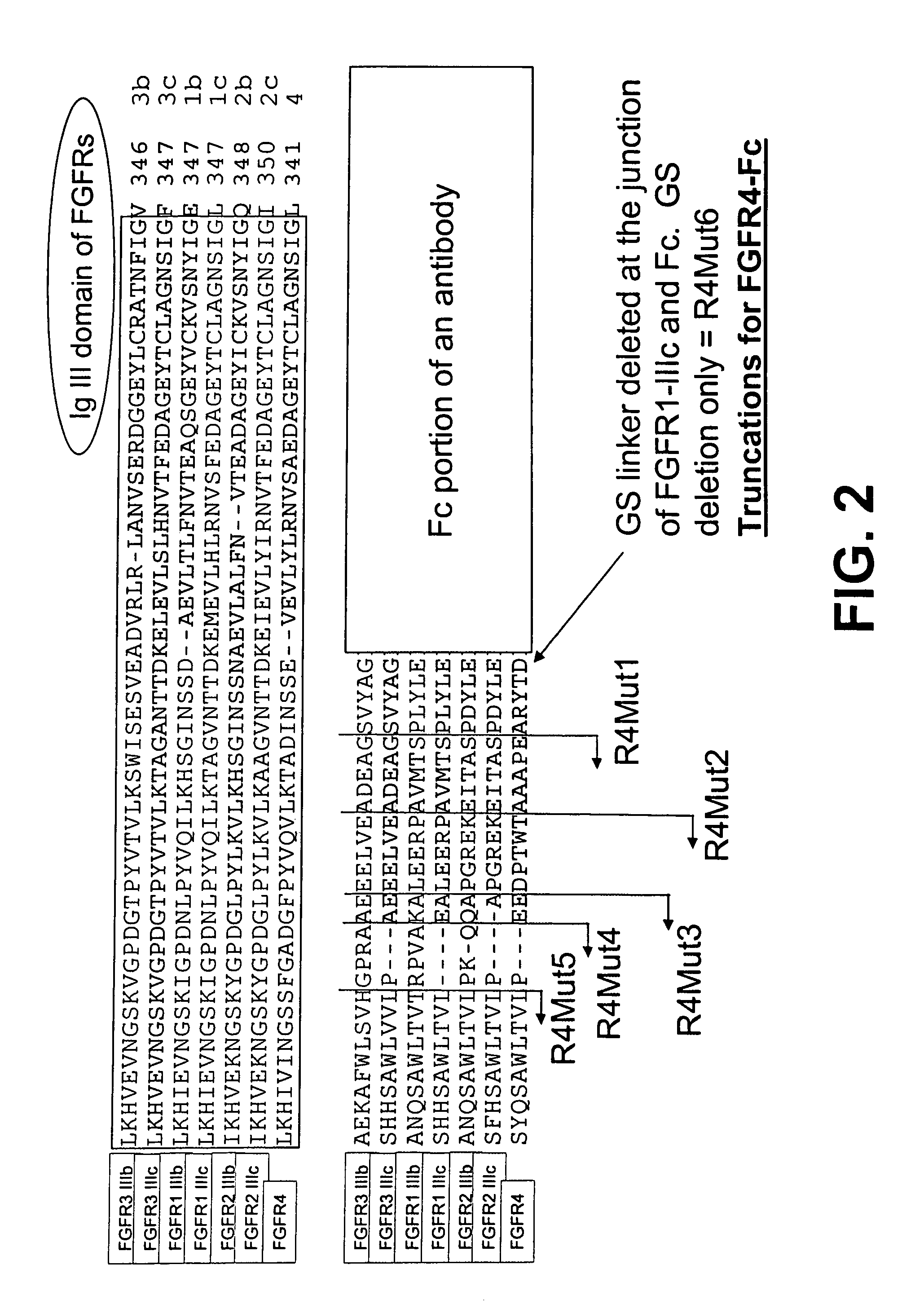
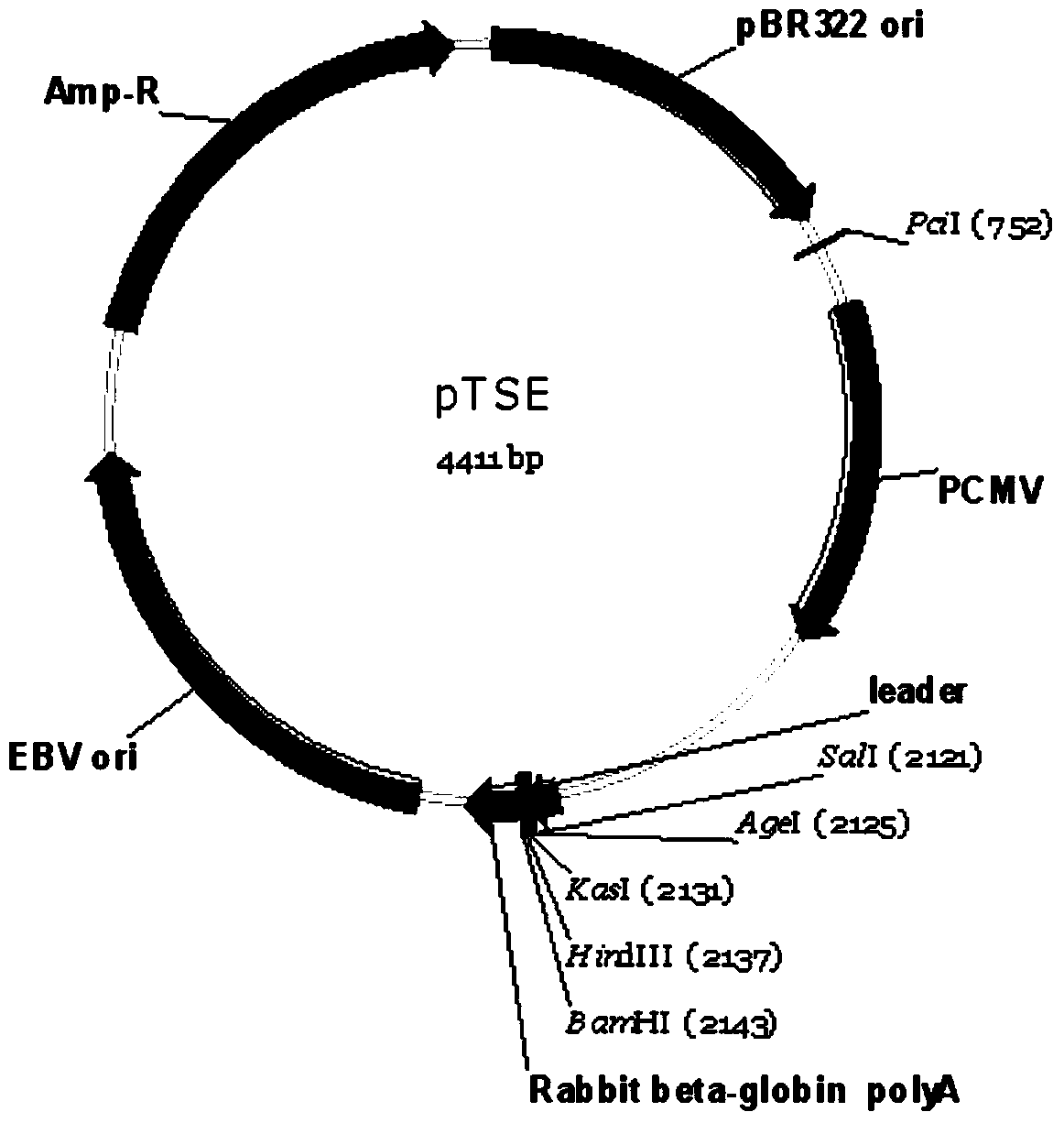
The invention provides FGFR fusion proteins, methods of making them, and methods of using them to treat proliferative disorders, including cancers and disorders of angiogenesis. The FGFR fusion molecules can be made in CHO cells and may comprise deletion mutations in the extracellular domains of the FGFRs which improve their stability. These fusion proteins inhibit the growth and viability of cancer cells in vitro and in vivo. The combination of the relatively high affinity of these receptors for their ligand FGFs and the demonstrated ability of these decoy receptors to inhibit tumor growth is an indication of the clinical value of the compositions and methods provided herein.
Owner:FIVE PRIME THERAPEUTICS
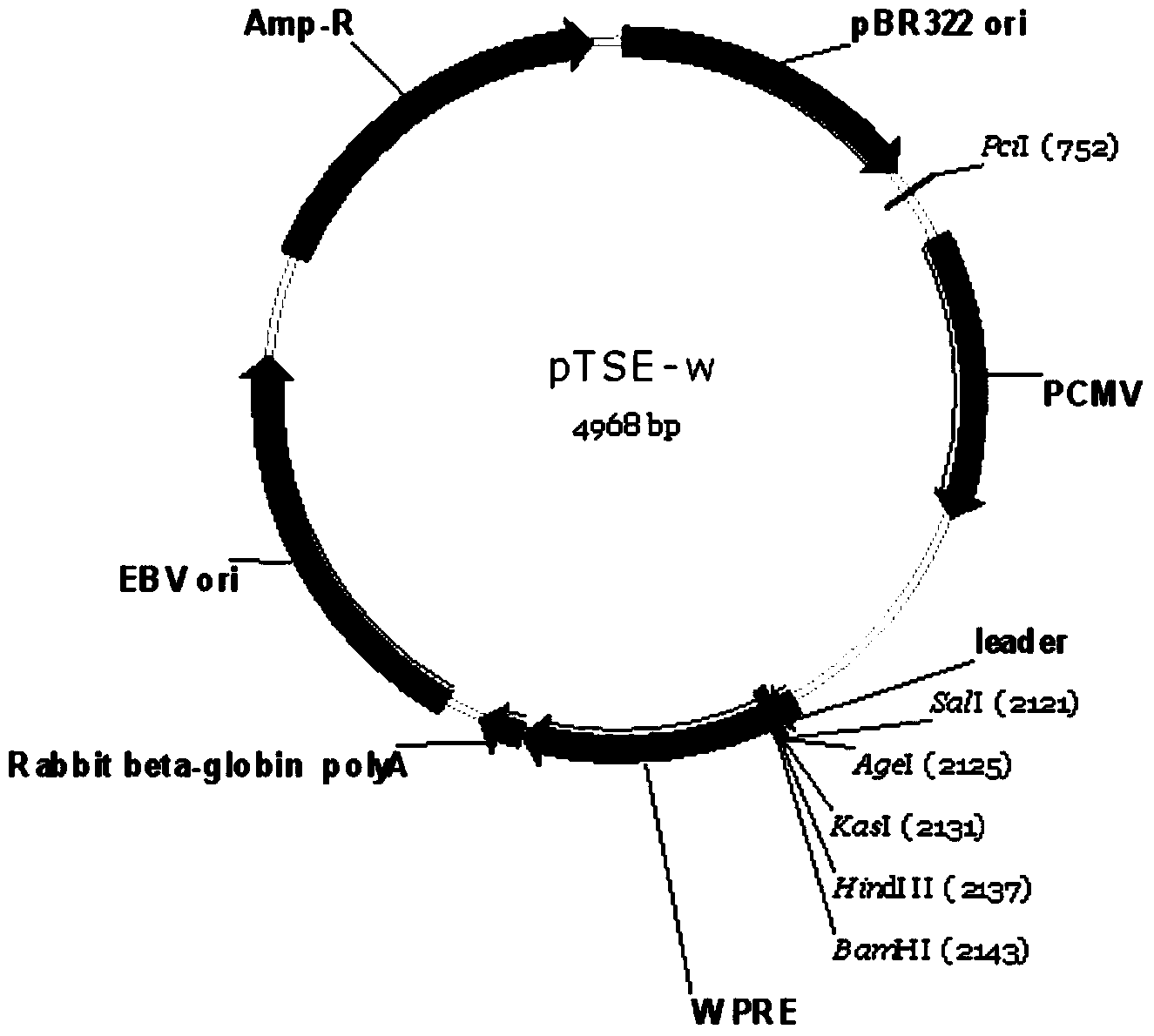
Construction and application of mammal cell high-efficiency expression vector
ActiveCN103525868AReduced transcript levelsShorten the development cycleVector-based foreign material introductionForeign genetic material cellsCloning SiteDeletion mutation
The invention relates to a mammal cell high-efficiency expression vector pSNEO. The vector is constructed on the basis of neomycin resistance screening gene NEO by combined strategy of weakening screening gene expression and reinforcing target gene expression. The screening gene weakening comprises the following two sides: introducing deletion mutant into the promoter to implement transcription weakening of the screening gene, and introducing a hairpin structure sequence before the translation initial site of the screening gene to implement the translation weakening of the screening gene. The expression reinforcement of the target gene is implemented by adding a transcription control element WPRE sequence between polyclone site and polyA tail of the target gene expression frame. The pSNEO vector can conveniently and quickly complete the construction of the stable high-expression cell strain of the target gene, and provides a new tool for preparing the high-polymer recombinant protein.
Owner:BIOTECH PHARMA CO LTD
Compositions and Methods of Treating Disease with Fgfr Fusion Proteins
InactiveUS20080171689A1Less susceptible to cleavageSenses disorderPeptide/protein ingredientsCancer cellEphA Receptors
The invention provides FGFR fusion proteins, methods of making them, and methods of using them to treat proliferative disorders, including cancers and disorders of angiogenesis. The FGFR fusion molecules can be made in CHO cells and may comprise deletion mutations in the extracellular domains of the FGFRs which improve their stability. These fusion proteins inhibit the growth and viability of cancer cells in vitro and in vivo. The combination of the relatively high affinity of these receptors for their ligand FGFs and the demonstrated ability of these decoy receptors to inhibit tumor growth is an indication of the clinical value of the compositions and methods provided herein.
Owner:FIVE PRIME THERAPEUTICS
Method for transforming rice into fragrant rice rapidly
InactiveCN105543228AGenetic stabilityRapid homozygousOxidoreductasesFermentationSelfingGene silencing
The invention discloses a method for transforming rice into fragrant rice rapidly. By means of the CRISPR / Case technology and related gene sequences in the rice fragrance metabolic process, a carrier is designed at the specific site, the element is transferred to non-fragrant rice through agrobacterium-mediated transformation, the gene is made to cause deletion mutation at the position of a fixed point, the gene is silent, fragrance cannot be normally metabolized and greatly accumulated, common rice is transformed into fragrant rice, then genetic transformation gene segments are separated from edited genes through selfing or hybridization, and the fragrant rice with the isozygoty capable of being stably inherited is rapidly obtained.
Owner:NINGXIA ACADEMY OF AGRI & FORESTRY SCI
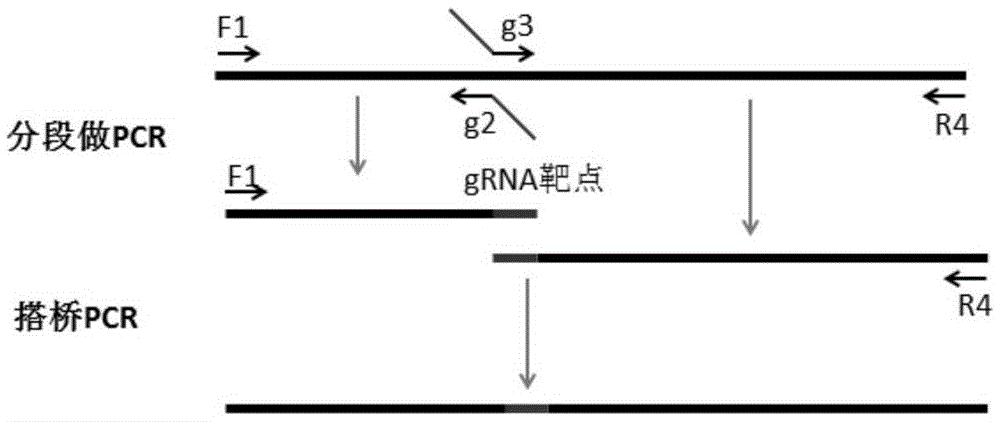
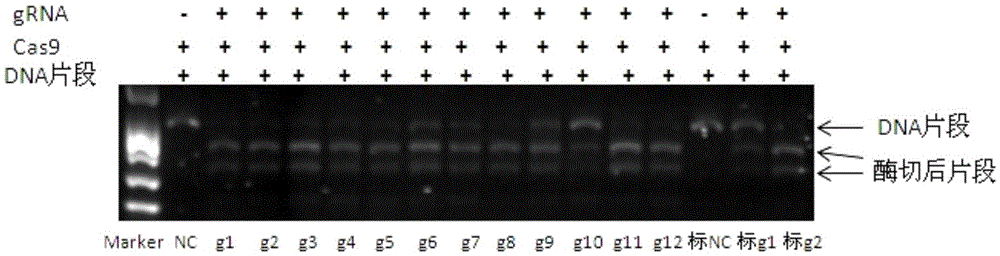
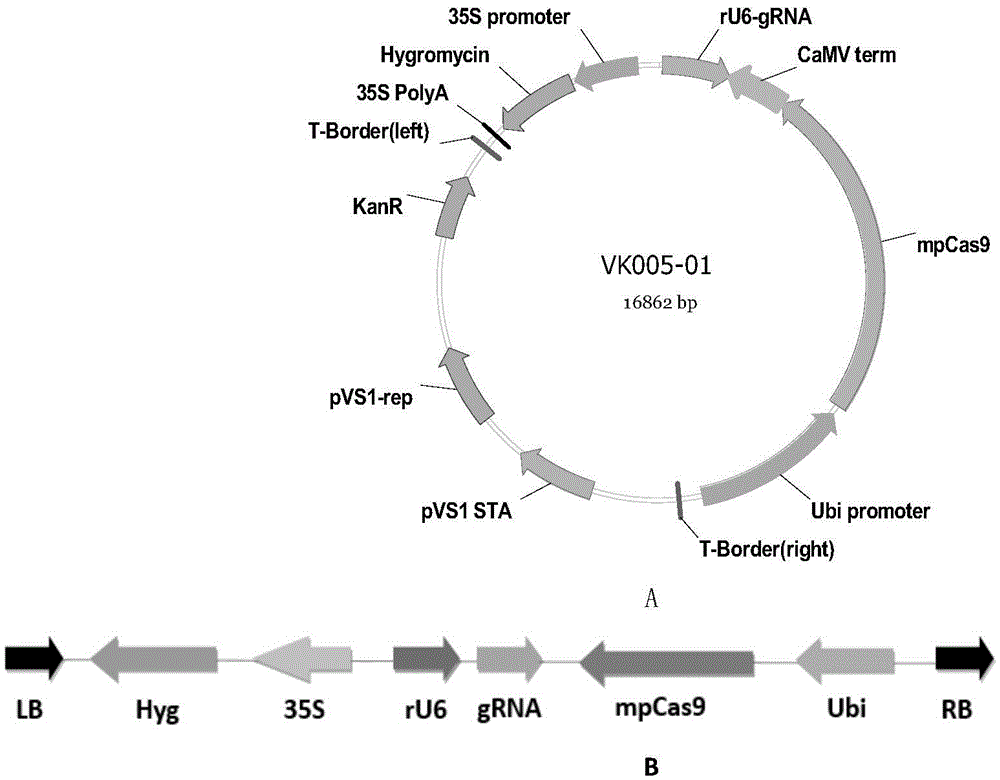
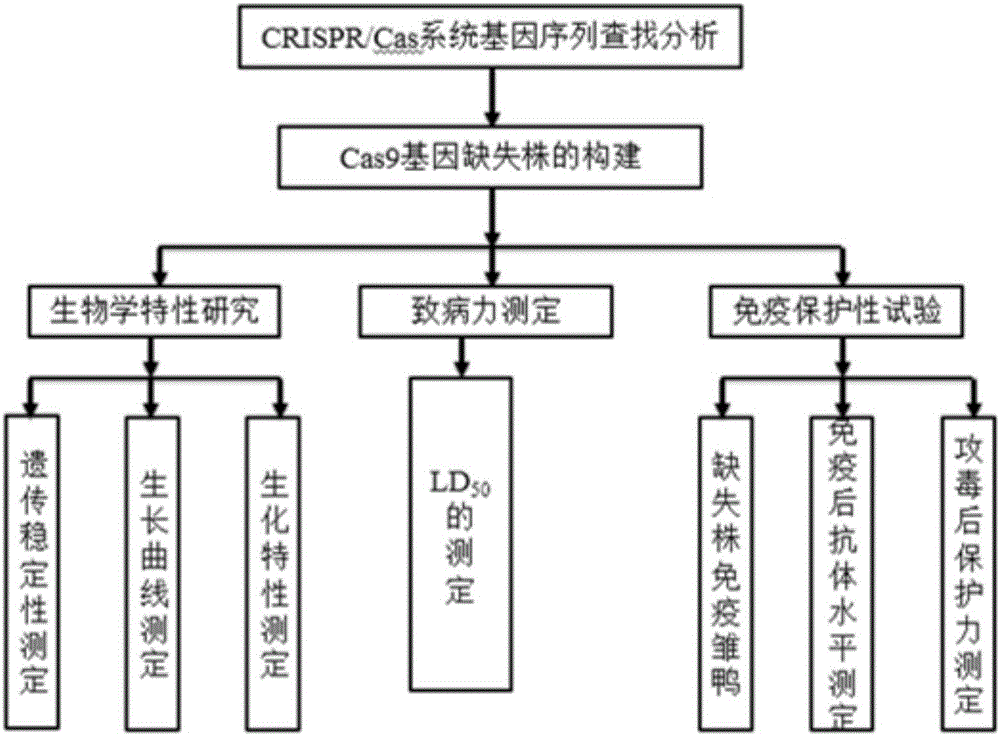
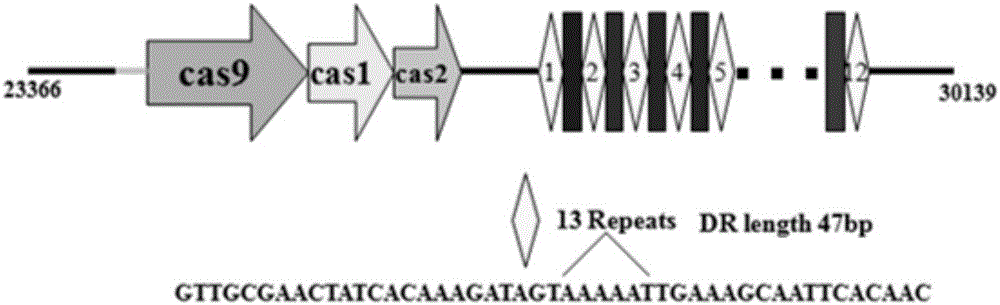
Riemerella anatipestifer mutant strain with Cas9 gene deletion and applications of riemerella anatipestifer mutant strain
InactiveCN106190903ALow toxicityReduced toxicityAntibacterial agentsBacterial antigen ingredientsCRISPRBacterial strain
The invention discloses a riemerella anatipestifer mutant strain with Cas9 gene deletion and applications of the riemerella anatipestifer mutant strain, belonging to the technical field of preparation of animal genetically engineered vaccines. The strain is preserved in the China Center for Type Culture Collection (CCTCC), and is assigned with the accession number of CCTCC NO:M 2016247. According to the strain, 2070bp of the Cas9 gene of the CRISPR-Cas system is deleted, so that the toxicity of the bacterial strain is obviously reduced, but the good immunogenicity is still reserved. Compared with the traditional inactivated vaccines, the strain with the Cas9 gene deletion has the advantages that the culture method is simple, the production cost is low, the inoculation can be carried out through the manners of nasal inhalation, mist spraying and the like, the operation is simple and rapid, the body can be stimulated to generate effective mucosal immunity, and meanwhile, the systemic immunity of the body also can be stimulated.
Owner:HUAZHONG AGRI UNIV
Breeding method capable of changing glume color of rice varieties with yellow glume to brownness
ActiveCN103981212AShort breeding cycleHorticulture methodsPlant tissue cultureChaffDeletion mutation
The invention provides a breeding method capable of changing the glume color of rice varieties with yellow glume to brownness. The breeding method comprises the following steps: selecting a target fragment in an extron region of a glume color determining gene OsCAD2, constructing a plant CRISPR / Cas9 targeting recombinant vector, guiding the recombination vector into a rice cell, regenerating the rice cell into seedlings, and sequencing genome target fragments of regenerated strains so as to obtain the strains, two allelic genes OsCAD2 of which simultaneously suffer function deletion mutation. Through phenotype identification, the change of the glume color is realized. Experiments prove that the breeding method can quickly change the glume color of the rice varieties.
Owner:RICE RES ISTITUTE ANHUI ACAD OF AGRI SCI
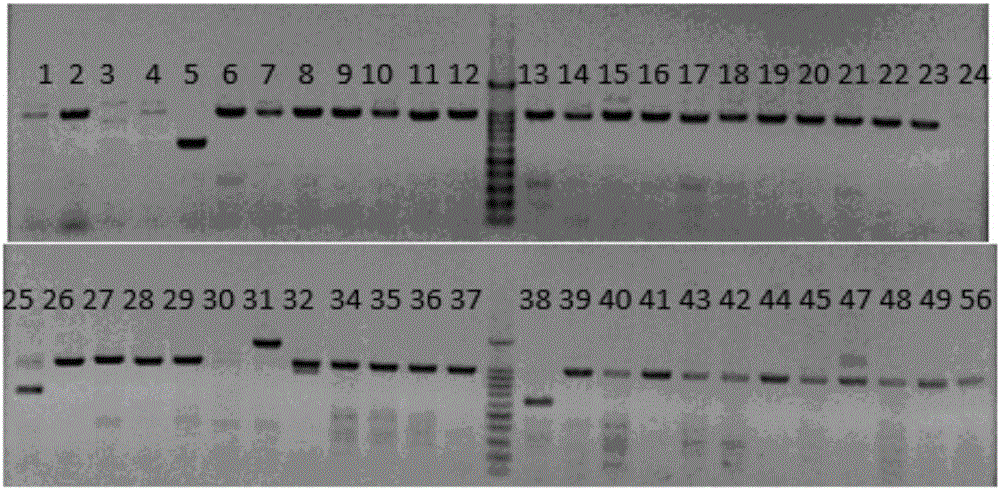
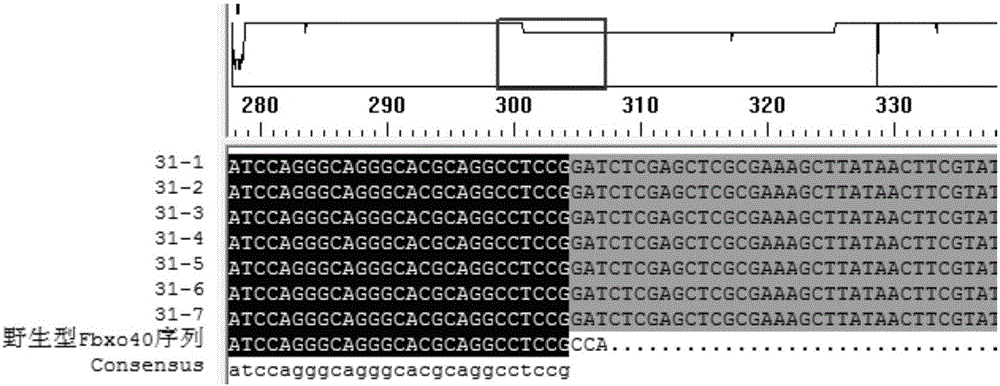
Production method for Fbxo40 gene knockout pigs
InactiveCN105821049AConvenient researchLow costNucleic acid vectorFermentationDiseaseAgricultural science
The invention provides a production method for Fbxo40 gene knockout pigs. The production method comprises the steps that a CRISPR-Cas9 targeting vector and a PGK-Neo resistant gene are jointly transfected into a porcine embryonic fibroblast to obtain a G418-resistant positive monoclonal cell, an Fbxo40 gene in the positive monoclonal cell generates insertion or deletion mutation, a reading frame generates frame shift and stops in advance, then the obtained cell is cloned to serve as a donor cell for nuclear transfer, and an oocyte serves as a receptor oocyte for nuclear transfer; cloned embryos are obtained through a somatic cell nuclear transfer technique, the high-quality cloned embryos are transferred into the fallopian tube of an oestrous sow, and the Fbxo40 gene knockout pigs are obtained through whole-period development. According to the production method, the Fbxo40 knockout pigs are efficiently obtained through a CRISPR-Cas9 gene editing technique at the low cost, and animal models are supplied to research of muscle development and diseases related to the muscles.
Owner:CHINA AGRI UNIV
Attenuated Pasteurella piscicida vaccine for fish
Live-attenuated vaccines against Edwardsiella ictaluri or against Pasteurella piscicida are disclosed. Both vaccines are incapable of reversion to virulence, because both are made by deletion mutations in the aroA gene, the purA gene, or both. These vaccines may be used not only to vaccinate fish against Edwardsiella ictaluri or Pasteurela piscicida, but also to serve as vectors to present antigens from other pathogens to the fish, thereby serving as vaccines against other pathogens as well, with no risk of infection by reversion to the virulent form of the pathogen in which the antigen occurs naturally.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
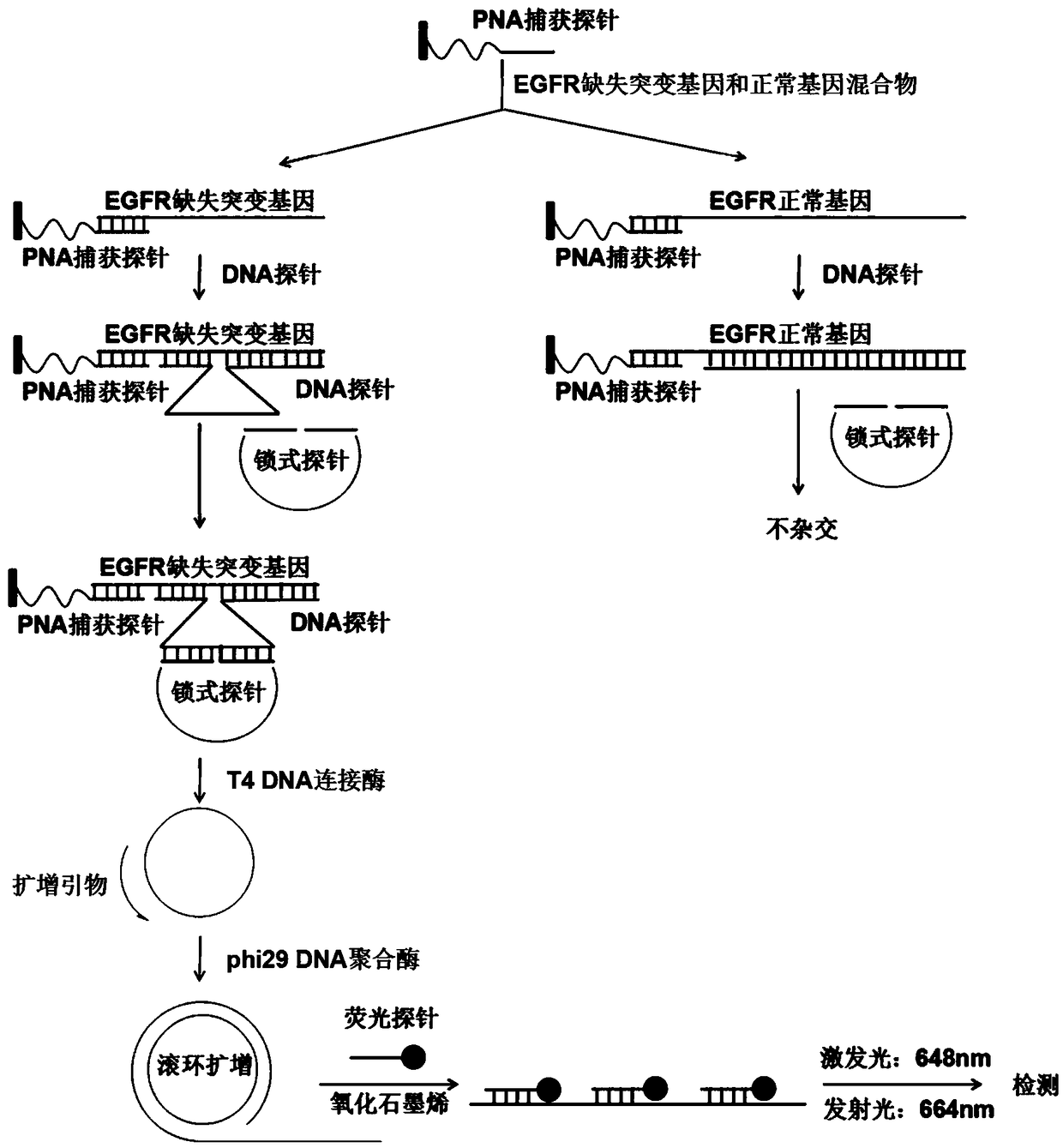
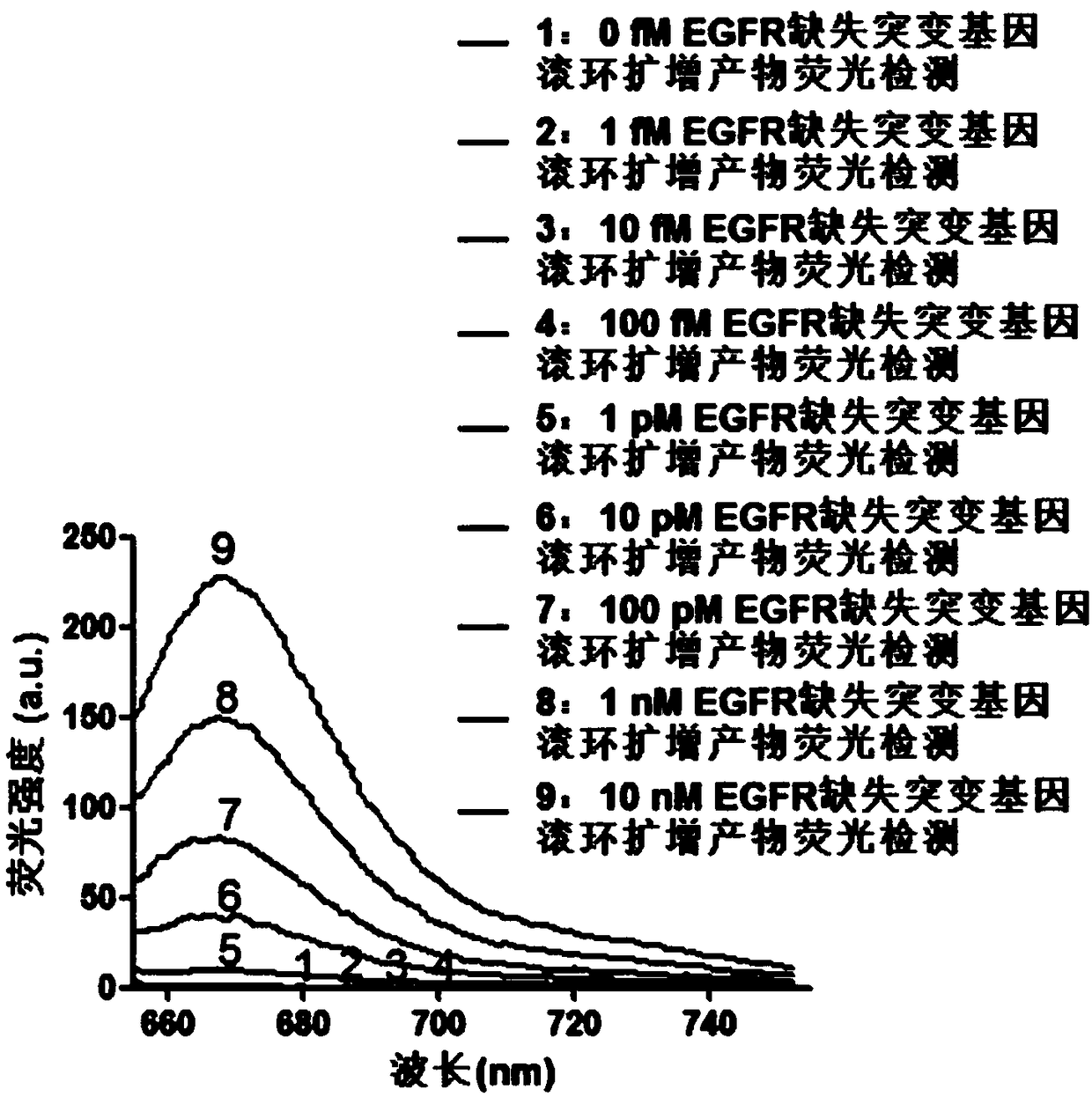
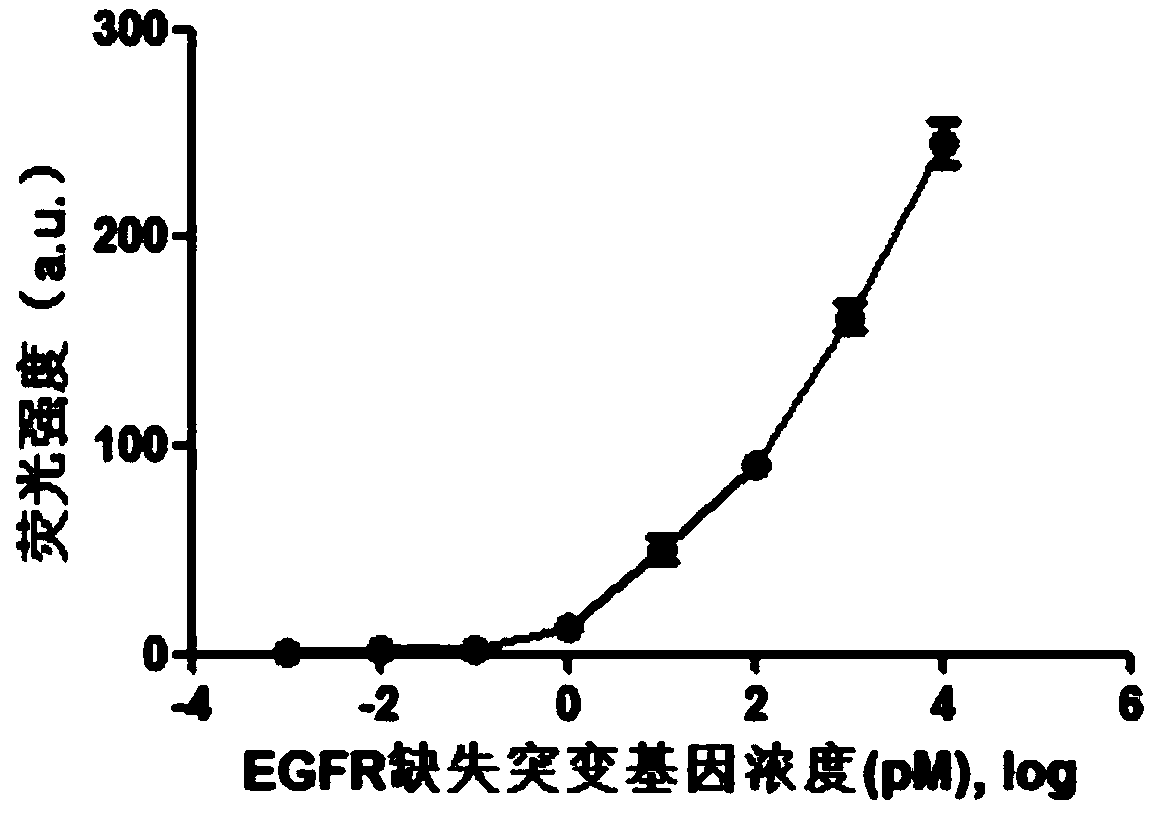
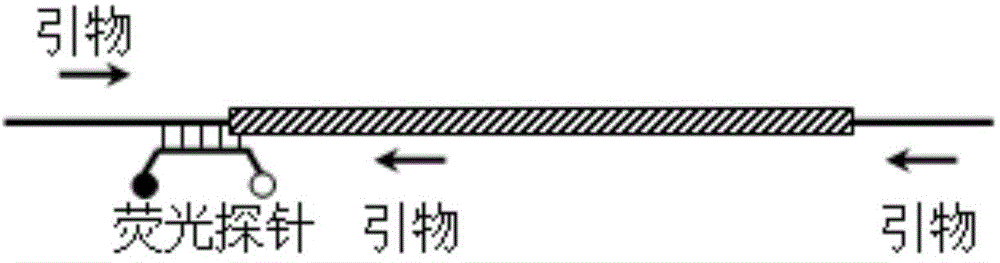
Fluorescence detection kit and fluorescence detection method for deletion mutation of gene
ActiveCN108949924AHigh sensitivityStrong specificityMicrobiological testing/measurementPolymerase LA-DNA
The invention provides a fluorescence detection kit for a deletion mutation gene. The kit includes: a PNA capture probe, a DNA probe, a padlock probe, a DNA polymerase, a DNA ligase, a rolling circleamplification primer, and a fluorescence probe. The invention also provides a method of using the kit to perform the fluorescence detection on the deletion mutation gene, wherein the method includes:1) immobilizing the PNA capture probe on the bottom of a pore plate, and hybridizing the probe with a target gene with a buffer solution; 2) hybridizing the target gene immobilized on the bottom of the pore plate with the DNA probe; 3) hybridizing a single chain section on the DNA probe, which is hybridized with the target gene, with the padlock probe, cyclizing the hybridized product with the DNAligase, and performing rolling circle amplification under effect of the rolling circle amplification primer and the DNA polymerase; 4) hybridizing the fluorescence probe with a rolling circle amplification product, quenching background fluorescence, and detecting the change on fluorescence intensity.
Owner:CIXI INST OF BIOMEDICAL ENG NINGBO INST OF MATERIALS TECH & ENG CHINESE ACAD OF SCI +1
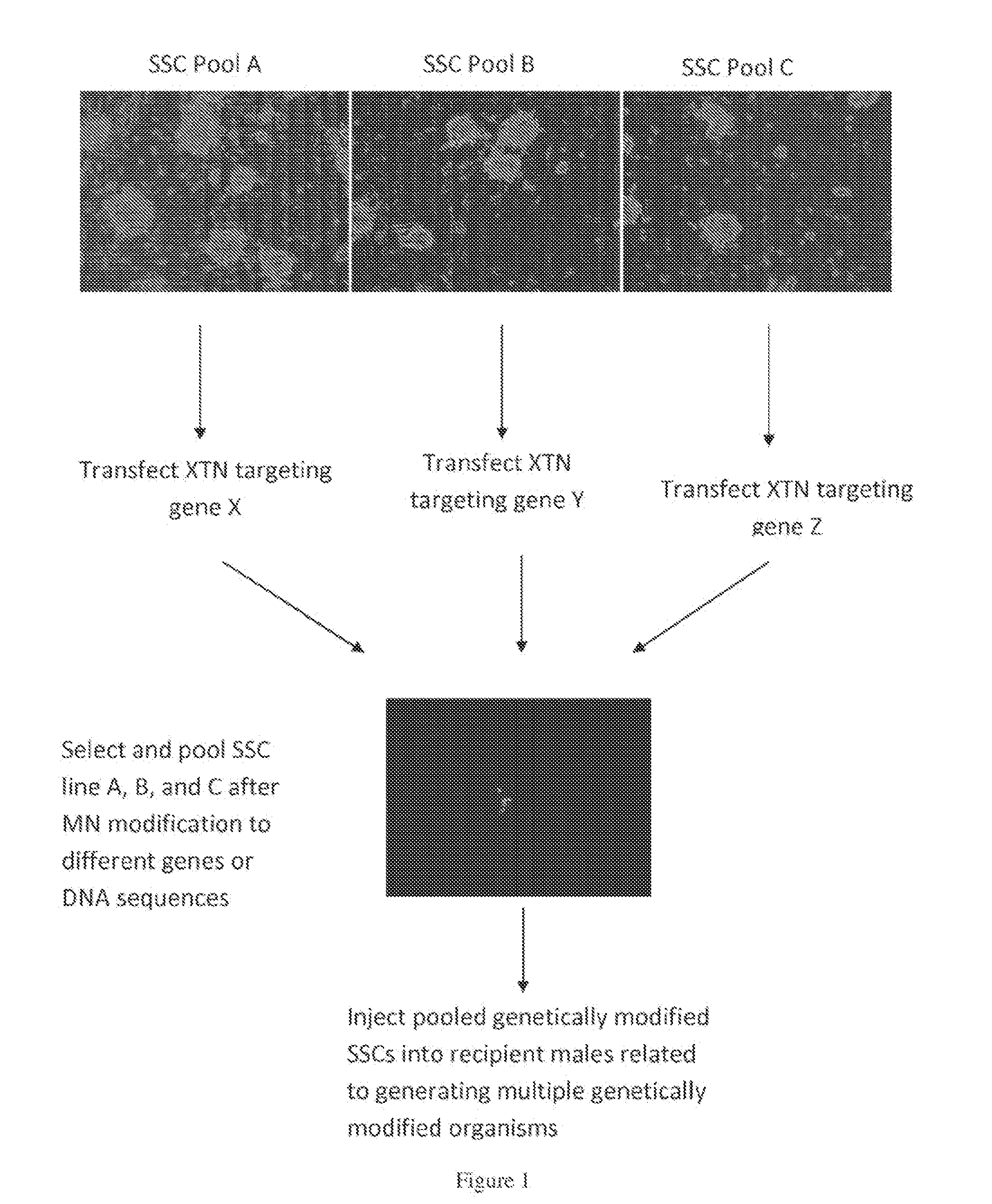
Methods for site-specific genetic modification in stem cells using xanthomonas tal nucleases (XTN) for the creation of model organisms
InactiveUS20140201858A1FermentationVector-based foreign material introductionHeterologousXanthomonas campestris
The invention relates to organisms and compositions comprising one or more stem cells or one or more embryos, wherein the one or more stem cells or one or more embryos comprise one or more of the following mutations: (i) a deletion mutation; (ii) a knockout mutation; and / or (iii) an addition of a heterologous nucleic acid sequence; wherein the one or more mutations of (i), (ii), and / or (iii) are site-specific mutations caused by a Xanthomonas TAL nuclease (XTN). The invention also relates to method of mutating an embryo, iPS cell, stem cell, or more particularly a spermatogonial stem cell by exposing the nucleic acid sequence contained within such embryos or cell with a Xanthomonas TAL nuclease.
Owner:TRANSPOSAGEN BIOPHARM +1
Yeast with increased butanol tolerance involving a multidrug efflux pump gene
ActiveUS20100221801A1Eliminate expression of ATP-bindingImprove toleranceFungiSugar derivativesYeastIncreased tolerance
Increasing tolerance to butanol in yeast has been accomplished by decreasing activity of Pdr5p encoded by an endogenous PDR5 gene. A deletion mutation of the PDR5 gene led to improved growth yield in the presence of butanol. Yeast cells with reduced Pdr5p activity, or other multidrug resistance ATP-binding cassette transporter protein activity encoded by CDR1 or BFR1, and a butanol biosynthetic pathway may be used for improved butanol production
Owner:GEVO INC
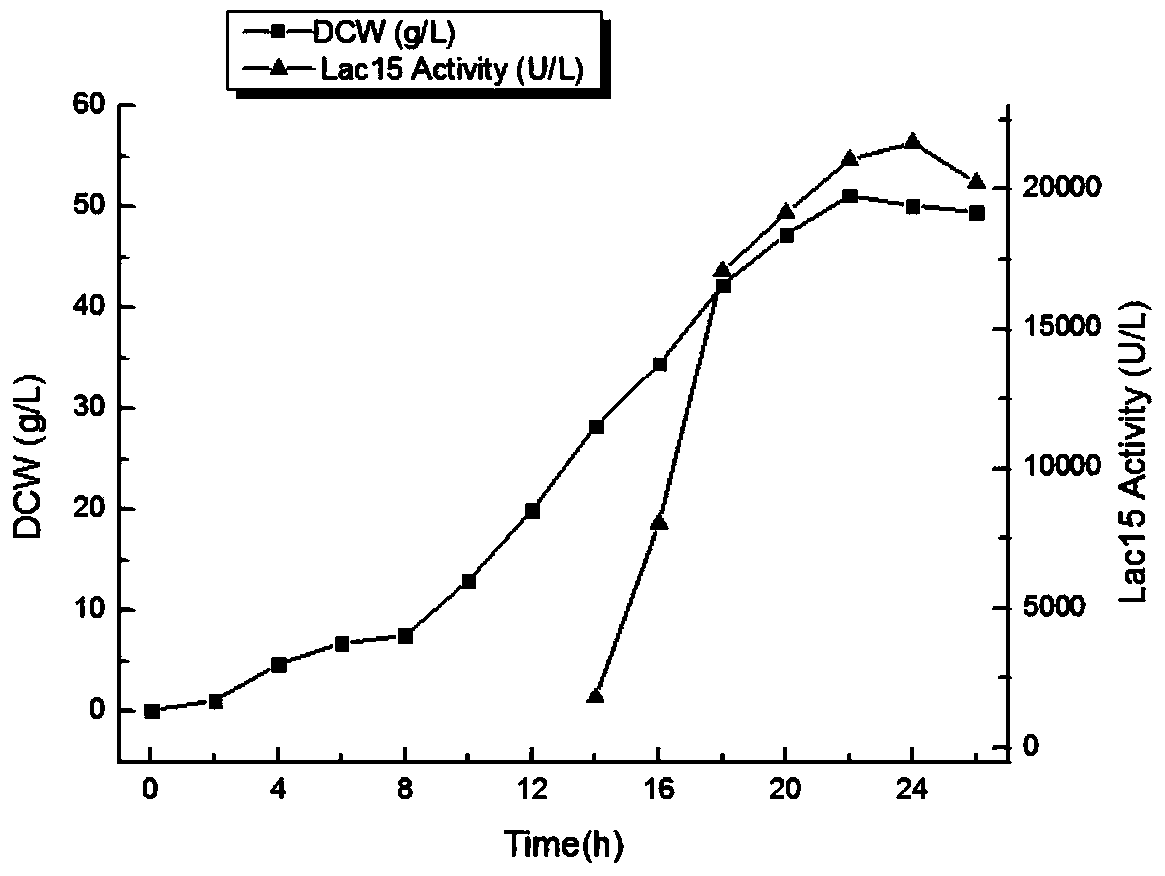
Bacterial laccase mutant protein, recombinant expression plasmid, transformed engineered strain and fermentation preparation method thereof
ActiveCN104087560AImprove stabilityIncrease temperatureBacteriaMicroorganism based processesMutated proteinGenetic engineering
The invention discloses a bacterial laccase mutant protein, which is characterized in that the mutant protein amino acid sequence is obtained by deletion mutation of the 323rd glycine residue to the 332rd glycine residue in the bacterial laccase amino acid sequence shown as SEQ ID No.1. Through a genetic engineering reconstruction method, a stability improved bacterial laccase protein coding gene, its expression plasmid and engineered bacteria can be obtained, and after large-scale fermentation and induced expression of the engineered bacteria, the stability improved bacterial laccase protein can be obtained. According to the invention, the marine uncultured microorganism source bacterial laccase Lac15 is taken as the foundation, and by means of genetic engineering reconstruction, mutant gene can be obtained. At the same time, a recombinant escherichia coli is employed to conduct high-density culture for high-efficiency expression of the bacterial laccase mutant protein. According to the invention, the stability and yield of the bacterial laccase are greatly improved.
Owner:ANHUI UNIVERSITY
Probes, method and chip for detecting alpha and/or beta-thalassemia mutation based on whole-gene capture sequencing and application of such probes, such method and such chip
ActiveCN106591441AEnables detection of deletions in large regionsMicrobiological testing/measurementDNA/RNA fragmentationBeta thalassemiaNew mutation
The invention provides primers, a method and a chip for detecting alpha and / or beta-thalassemia point mutation and deletion mutation based on whole-gene capture sequencing and application of such primers, such method and such chip. The primers, the method, the chip and application thereof have the advantages that through designing of capture probes, relevant genes involved in alpha-thalassemia and beta-thalassemia are enriched and all mutation information including SNP and indel in full-length sequences of genes is detected; through addition of autosome, X-chromosome and Y-chromosome regions as well as upstream and downstream regions of coded genes as references, structure variations such as SNV and CNV are detected; compared with existing various hotspot mutation site detection technologies, the method is capable of detecting hotspot mutation information as well as some rare mutations and undiscovered new mutation types to detect and analyze full-length sequence specificity of target genes, fully covers the mutation types and makes up the defect that a conventional detection method easily causes missing detection of low-frequency mutations and rare mutations greatly.
Owner:SHENZHEN E GENE TECH
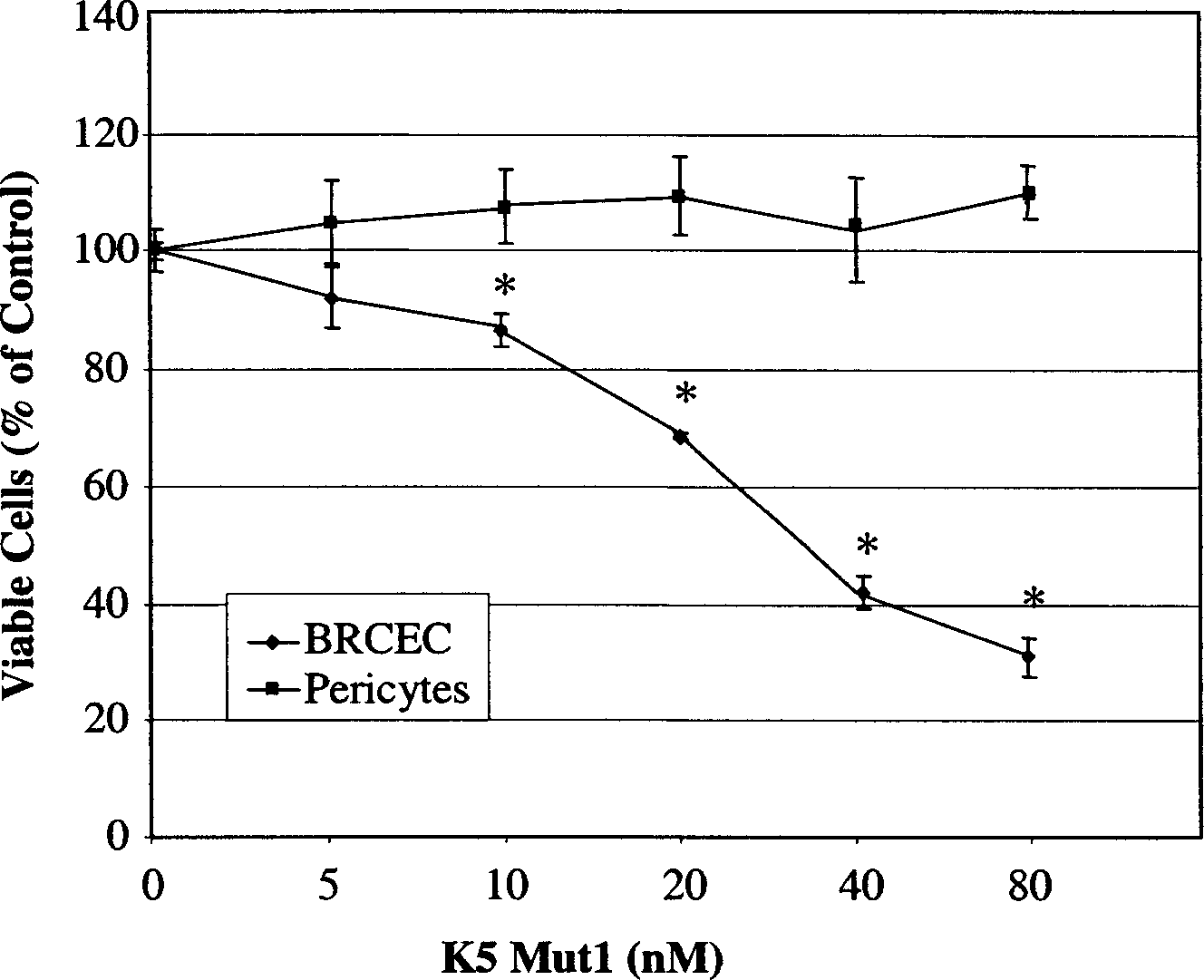
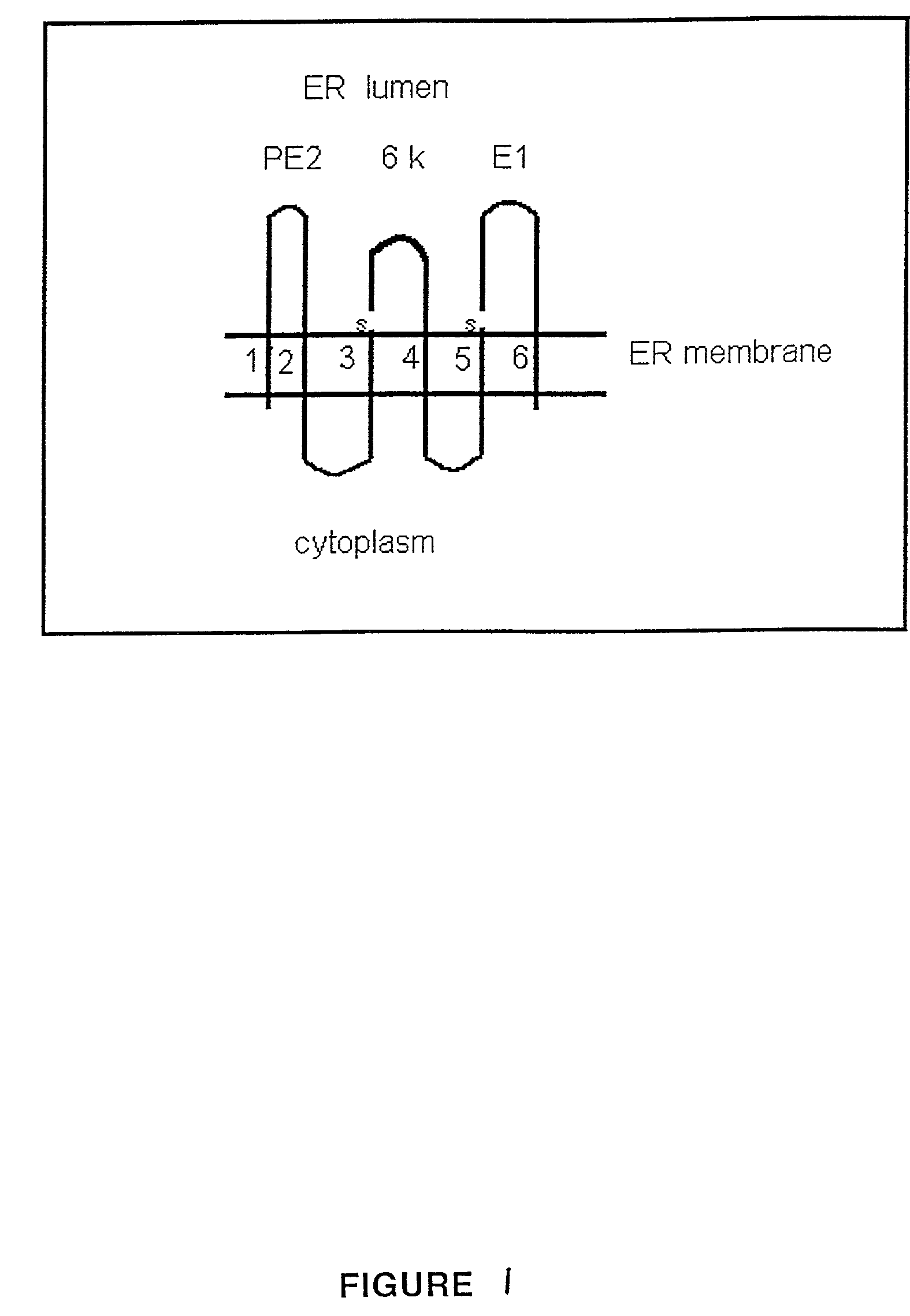
Human profibrinolysin kringle 5 deletion mutation recombinant polypeptide
InactiveCN1451746AReduce leakageHas a specific inhibitory effectBacteriaEnzymesProfibrinolysinEscherichia coli
A human plasminogen Kringle 5 deleted mutant recombinant polypeptide for treating the retinopath, such as vascular hyperplasia and angiorrhea, is prepared through removing 15 amino acid residue from K5 molecule while retaining 3 disulfide bond, expressing in colibacillus, and purifying by affinity chromatography.
Owner:QIYUAN BIOLOGICAL SCI & TECH GUANGZHOU CITY
Membrane virus host range mutations and their uses as vaccine substrates
InactiveUS7128915B2Lower levelSsRNA viruses positive-senseSugar derivativesViral VaccineTransmembrane domain
The present invention is directed to genetically engineered, membrane-enveloped viruses with deletion mutations in the protein transmembrane domains. Also provided are viral vaccines based on the engineered viruses, methods of producing and using such vaccines.
Owner:RES DEVMENT FOUND
Gene mutation detection method and apparatus
InactiveCN106566877AImprove realismImprove accuracyBioreactor/fermenter combinationsBiological substance pretreatmentsGenes mutationDeletion mutation
The invention discloses a gene mutation detection method and apparatus. The method comprises the following steps: 1, acquiring sequencing data of a sample to be detected and a contrast sample; and 2, judging that whether SNP mutation, InDel mutation and / or deletion mutation exists in the sequencing data of the sample to be detected: (1) carrying out homogenization processing, calculating a standard deviation and median, and calculating the irrelevance Z value according to a formula (1); and (2) judging deletion: judging the deletion mutation exists in the window of the sample to be detected if the Z value Z = (the homogenous sequence number of the sample to be detected - the median) / the standard deviation (1) is greater than 3. The method and the apparatus improve the detection flux and the detection accuracy.
Owner:天津诺禾致源生物信息科技有限公司
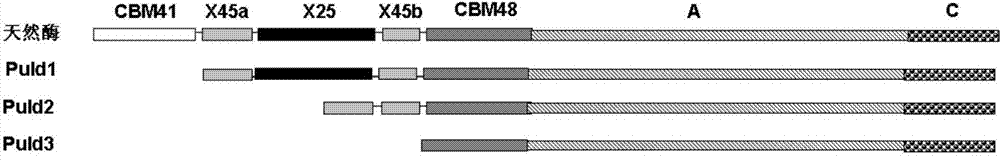
Pullulanase mutant with improved secretion efficiency and heat stability and preparation method of pullulanase mutant
ActiveCN103571812AImprove efficiencyImprove thermal stabilityFermentationVector-based foreign material introductionHeat stabilityPhysical chemistry
The invention discloses a pullulanase mutant with improved secretion efficiency and heat stability and a preparation method of the pullulanase mutant, and belongs to the field of gene engineering and enzyme engineering. The secretion efficiency and the heat stability of pullulanase are improved through structural domain deletion mutation; deleted structural domains are CBM41, X45 and X25 structural domains or combinations thereof; and the mutation technical scheme capable of improving the secretion efficiency and the heat stability of pullulanase is provided. The obtained pullulanase structural domain deletion mutant has at least one of changed properties as follows: 1), the extracellular secretion efficiency is improved after recombinant bacteria are fermented; and 2), and the heat stability is improved under the conditions of pH 4.0-5.0 and 60 DEG C. Compared with natural pullulanase, structural domain deletion mutants are more suitable for production, preparation and applications of the pullulanase.
Owner:JIANGNAN UNIV
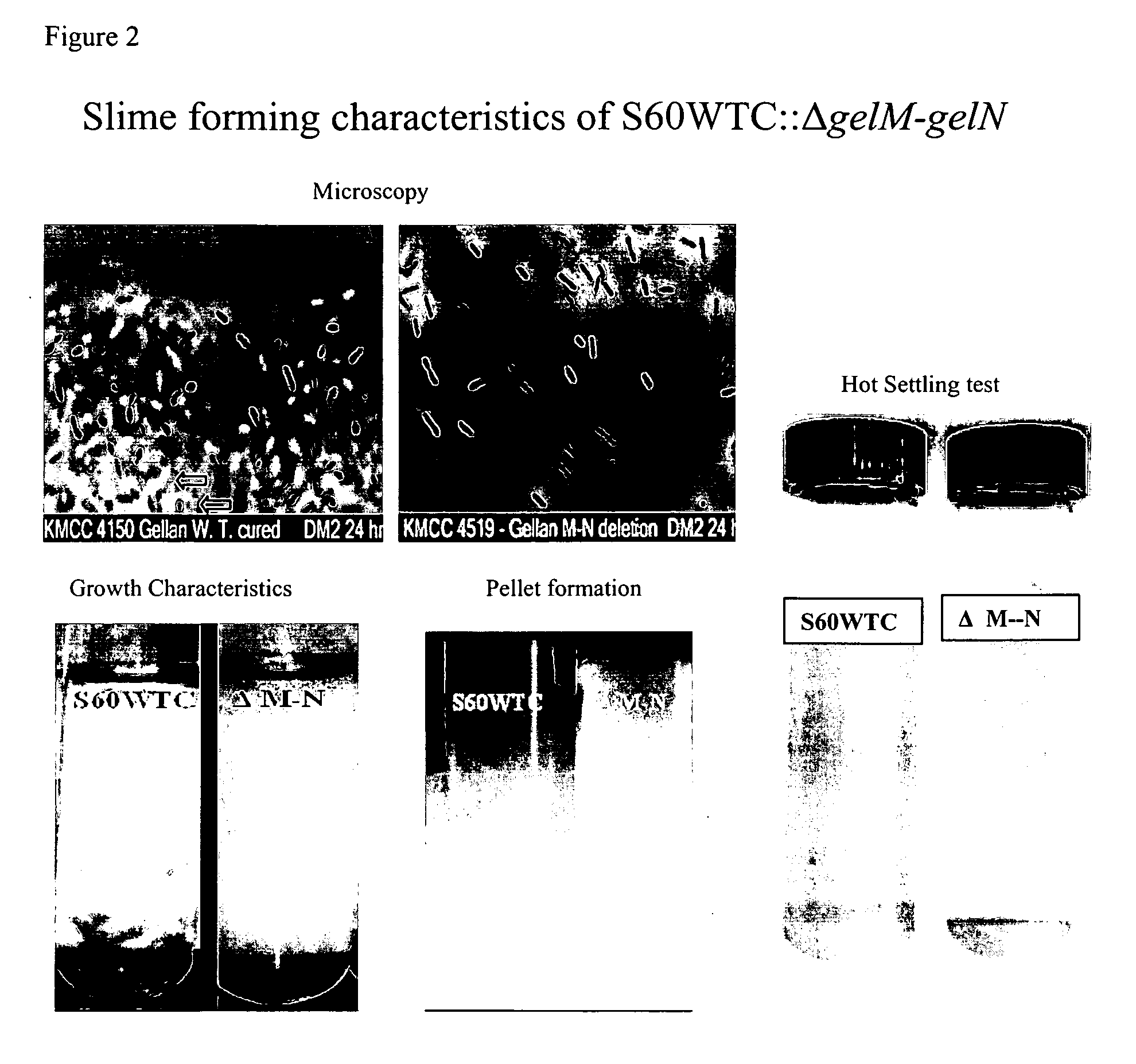
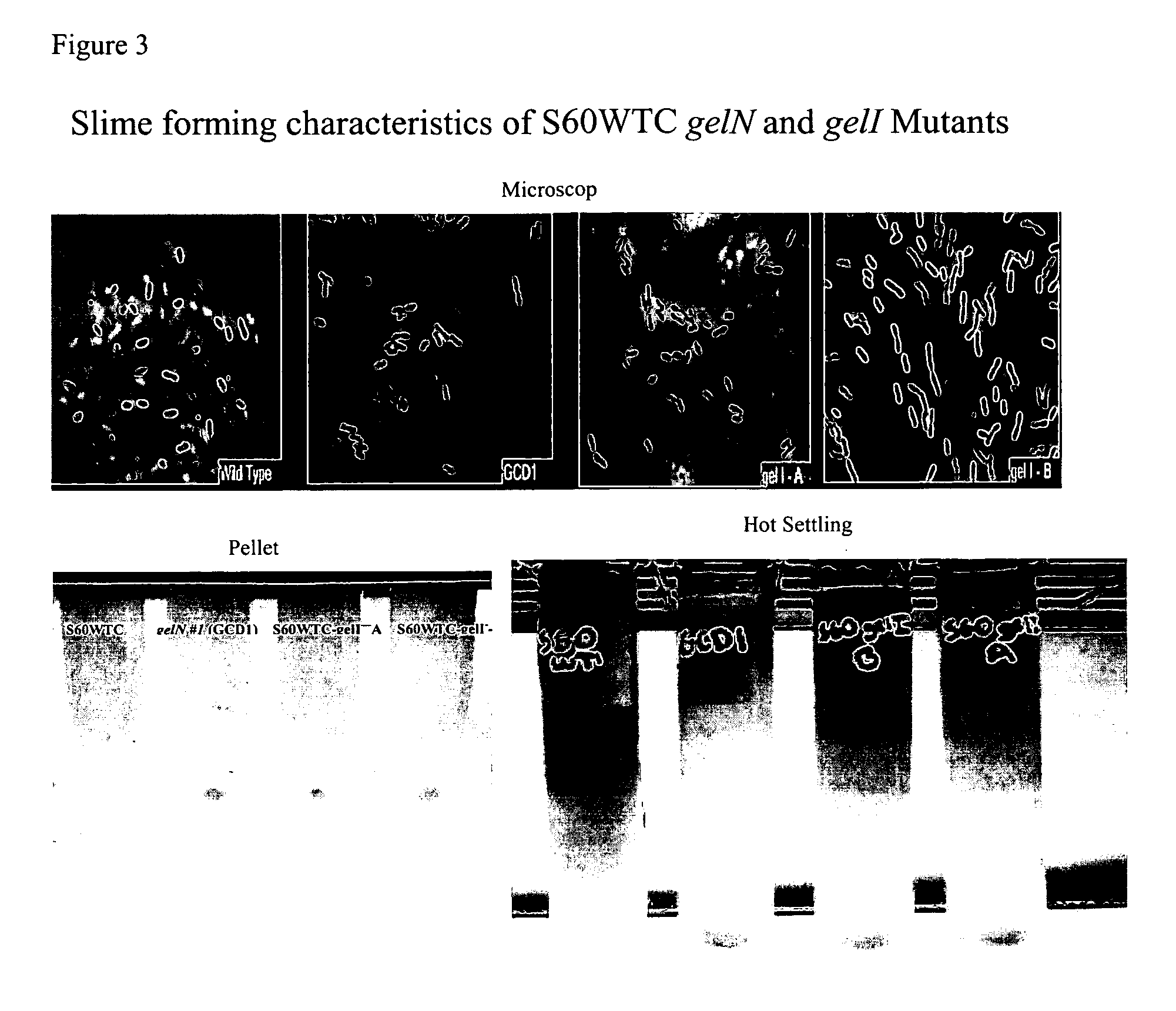
Targeted gene deletions for polysaccharide slime formers
Sphingomonas strains have extracellular polysaccharide (e.g., gellan, diutan) that is firmly attached to the cell surface. This attachment may limit polysaccharide production by impairing uptake of nutrients into the cell or due to limited sites for polysaccharide biosynthesis on the cell surface. Two genes for polysaccharide biosynthesis, designated gelM and gelN in gellan-producing strains and dpsM and dpsN in diutan-producing strains, have been inactivated by deletion mutations and shown to produce polysaccharide that is not firmly attached to the cell surface, i.e., slime form. Another gene for polysaccharide biosynthesis, designated gelI in gellan producing strains, was inactivated by insertion mutation and also shown to produce the slime phenotype. The homologous gene dpsi in the diutan producing strain should also be involved in the attachment of the polysaccharide to the cell surface. The slime characteristic was demonstrated by the ability of the cells to be centrifuged and the lack of cell clumping as seen under the microscope or in diluted suspensions. The diutan slime mutants had somewhat increased productivity and the recovered diutan product had significantly improved rheology. Gellan slime mutants had lower broth viscosity which facilitates mixing during fermentation; however, the recovered gellan product had lower gel strength than the gellan produced from a capsular strain. A deletion in a gene gelR, which encodes a protein with homology to surface proteins and outer membrane proteins and weak homology to proteins with polysaccharide degradation activity, was shown to restore higher gel strength to the slime form of gellan, and to produce gellan of higher gel strength than that of the capsular gellan producing strains.
Owner:CP KELCO U S INC
Composition for modifying nucleotide sequence, method and application
ActiveCN109517841AWide working windowReduce deletionVector-based foreign material introductionDNA preparationCytosine deaminaseNucleotide
The invention discloses a composition for modifying a nucleotide sequence, a method and application, and relates to the technical field of gene editing. The composition comprises a first vector and asecond vector, wherein the first vector is provided with the following expression elements, such as a cytosine deaminase expression element, an adenine deaminase expression element and a mutant Cas enzyme expression element; the second vector is provided with the following expression elements, such as a gRNA (guide ribonucleic acid) expression element and a uracil glycosidase inhibitor expressionelement. The composition is used for modifying the C bases at the 1st to 16th sites at the upstream of PAM (protospacer adjacent motif) of the target nucleotide sequence, so that the transformation ofC / G to T / A is realized. Compared with the existing gene editing technique, the composition and the method have the advantages that the working window is broader; the DSB (double-strand break) and indels (insertion / deletion mutations) are not introduced, the off-target effect is very low, the safety is higher, and the like.
Owner:EAST CHINA NORMAL UNIV +1
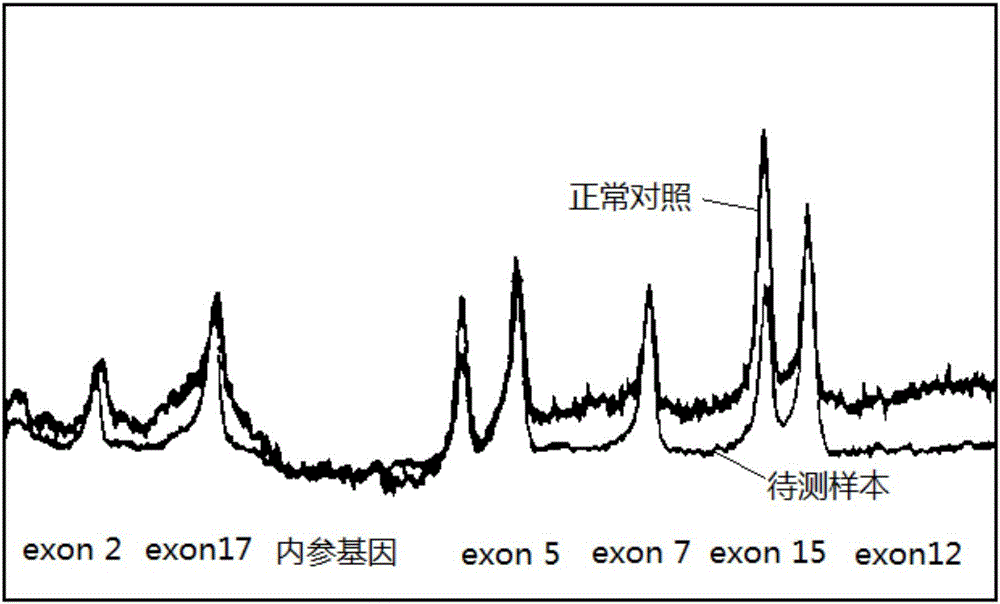
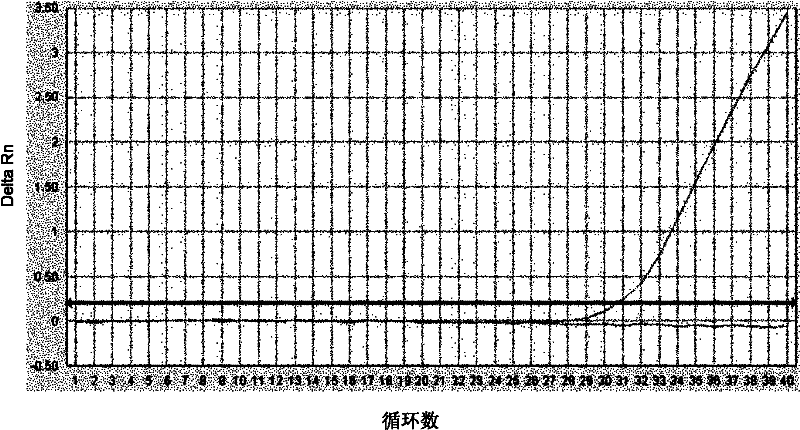
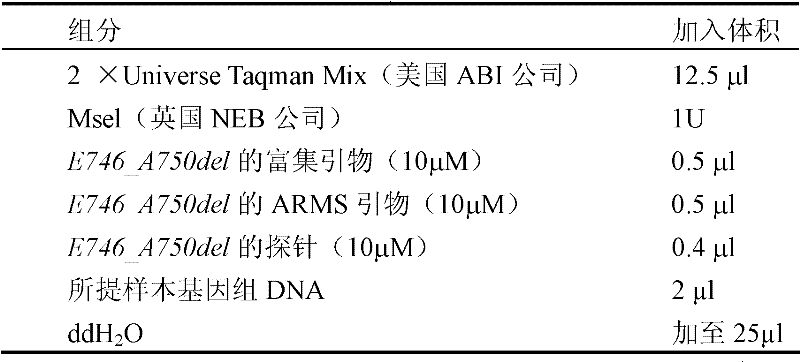
Method for detecting exon 19 deletion mutation and exon 21 point mutation of epidermal growth factor receptor gene
InactiveCN102220413AAchieve enrichmentReduce stepsMicrobiological testing/measurementFluorescence/phosphorescenceEnzyme digestionCuticle
The invention discloses a method for detecting exon 19 deletion mutation and exon 21 point mutation of an epidermal growth factor receptor gene. In method, enzyme digestion enrichment PCR (Polymerase Chain Reaction) reaction and ARMS (amplification refractory mutation system) fluorescence quantitative PCR reaction are carried out in one reaction system to respectively detect E746_A750del deletionmutation and L858R point mutation; and the enzyme digestion mutation enrichment PCR and ARMS fluorescent quantitative PCR are integrated together, the temperature and time are effectively controlled,and one reaction process is carried out to complete the enzyme digestion mutation enrichment and ARMS fluorescent quantitative PCR reaction.
Owner:BEIJING INST OF GENOMICS CHINESE ACAD OF SCI CHINA NAT CENT FOR BIOINFORMATION
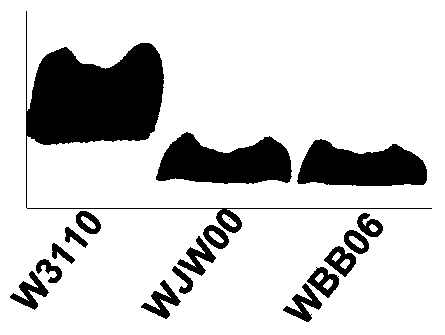
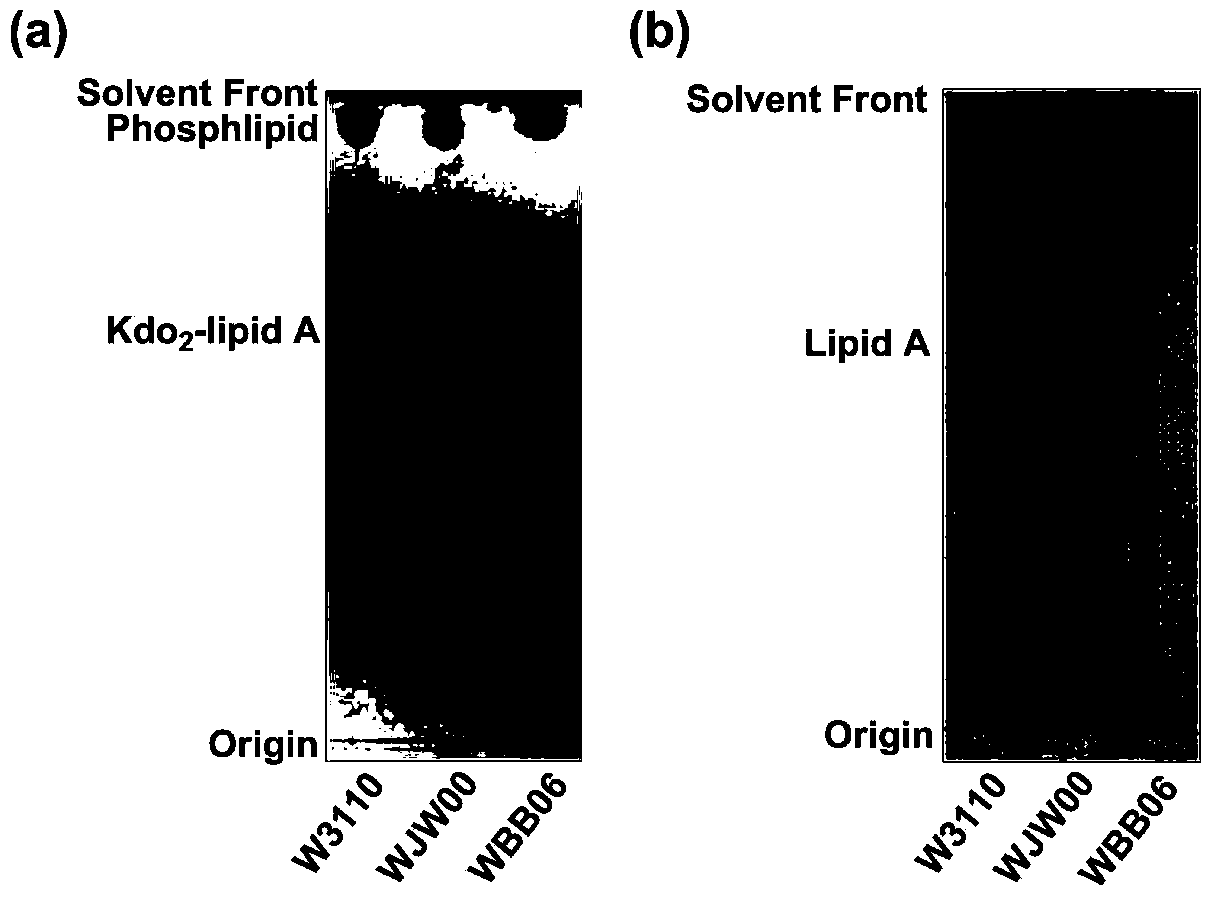
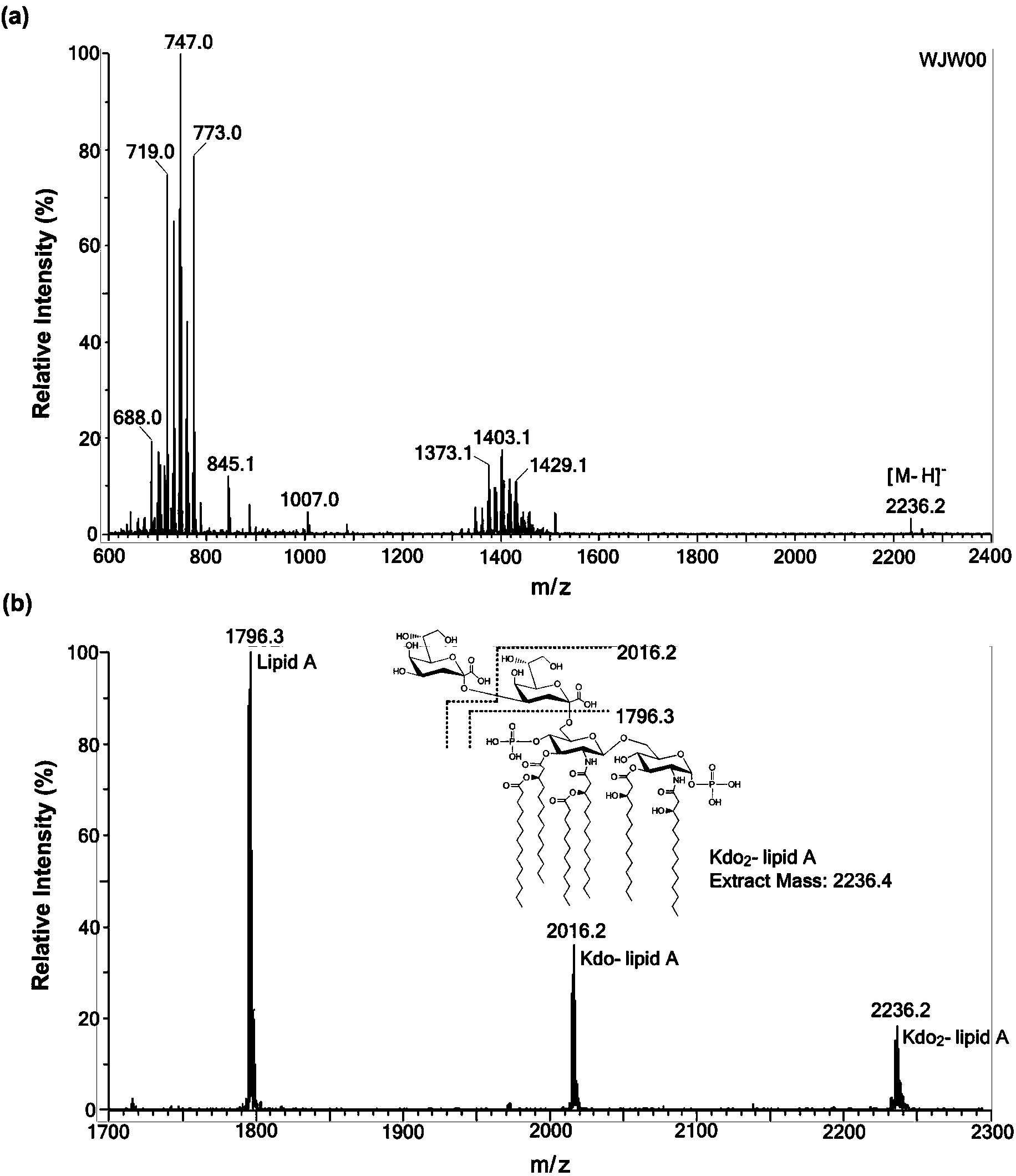
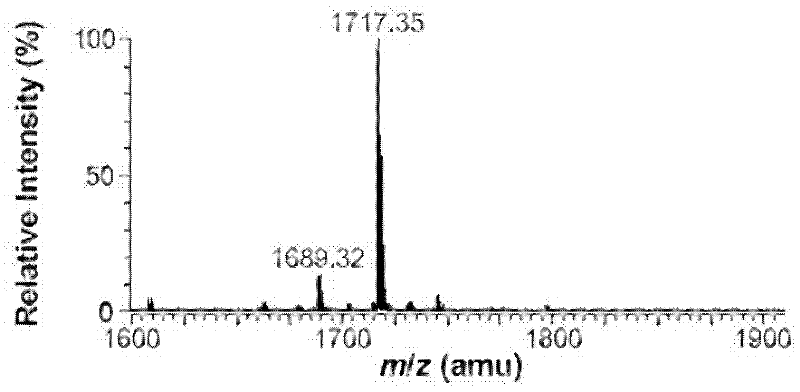
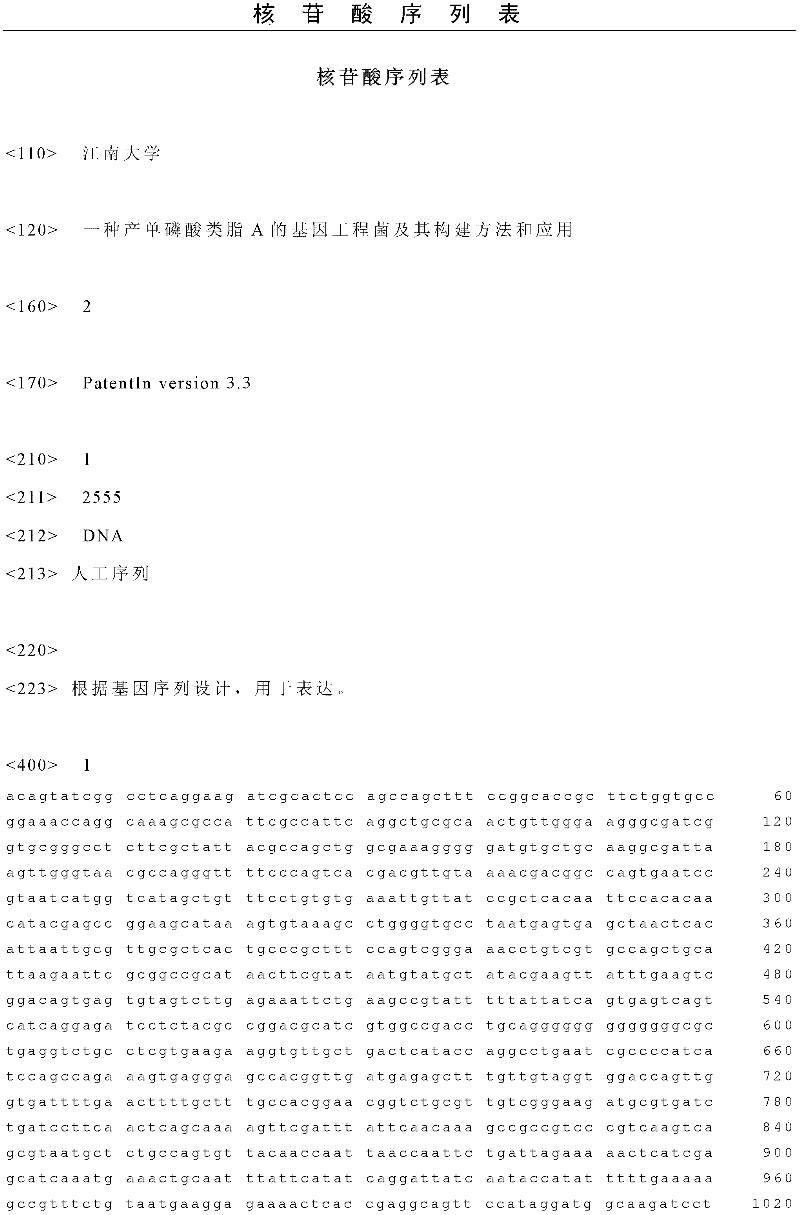
Gene engineering bacterium capable of producing Kdo2-lipid A, construction method and applications thereof
ActiveCN103820377ASingle structurePrecise structureBacteriaMicroorganism based processesResistant genesKdo2-lipid A
The invention discloses a gene engineering bacterium capable of producing Kdo2-lipid A, a construction method and applications thereof, and belongs to the field of genetic engineering. The gene engineering bacterium is WJW00, and the gene engineering bacterium is non-resistant E. coliW3110 delta rfaD, wherein the rfaD carries out deletion mutation and is inactivated. The bacterium strain Kdo2-lipid A has a right structure and good growth situation, moreover, not any external resistant gene sequence is introduced, so the bacterium strain is suitable for industrial massive production.
Owner:JIANGNAN UNIV
Gene for regulating and controlling corn drought stress resistance and application thereof
The invention relates to the technical field of plant genetic engineering, in particular to a gene for regulating and controlling the corn drought stress resistance. The gene is a ZmPP2CA10 deletion mutation gene; the deletion segment is a continuous base sequence with a distance being 301bp away from the upstream of the ZmPP2CA10 initial codon ATG; the continuous basic group sequence is shown as SEQ ID NO:1; the nucleotide sequence of the mutated ZmPP2CA10 promoter is shown as SEQ ID NO:2. The deletion mutation gene can be used for corn drought resistance relevant molecular marker auxiliary breeding and corn drought resistance auxiliary identification; the operation is convenient; the accuracy rate is high.
Owner:HUAZHONG AGRI UNIV
Gene for controlling rice fertility, encoded protein and application thereof
ActiveCN102634522AExpanding the Germplasm BaseSimple methodBacteriaMicrobiological testing/measurementAnti-Sense RNAGermplasm
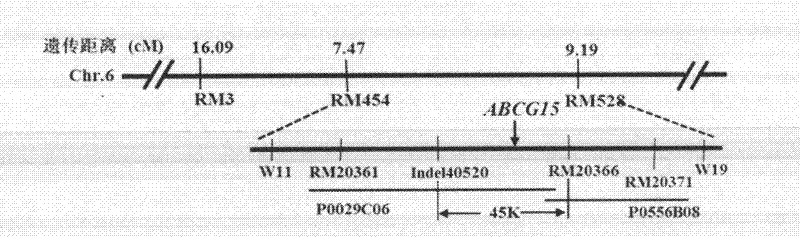
The invention discloses a gene ABCG15 for controlling rice fertility, an encoded protein and the application thereof. The invention also discloses a single recessive nuclear sterile gene, which is generated by mutation caused by deletion of the gene 12bp for controlling the rice fertility. The invention also discloses a method for obtaining a rice sterile line by controlling the expression of the rice fertility gene according to an RNAi technology and the method comprises the following steps of: amplifying a DNA fragment for generating an anti-sense RNA, constructing an expression vector, and transforming the DNA fragment into the normal fertile rice, so as to obtain a new rice nuclear sterile material. The newly found gene for controlling the rice fertility provides a new way for culturing the rice nuclear sterile line. The method for generating the rice nuclear sterile line is convenient and quick. The rice nuclear sterile material can be used for replacing the manual castration during the rice hybridization, labor is saved; the rice nuclear sterile material also can be used for recurrent selective breeding and plays an important role in widening the rice germ plasm basis.
Owner:SICHUAN AGRI UNIV
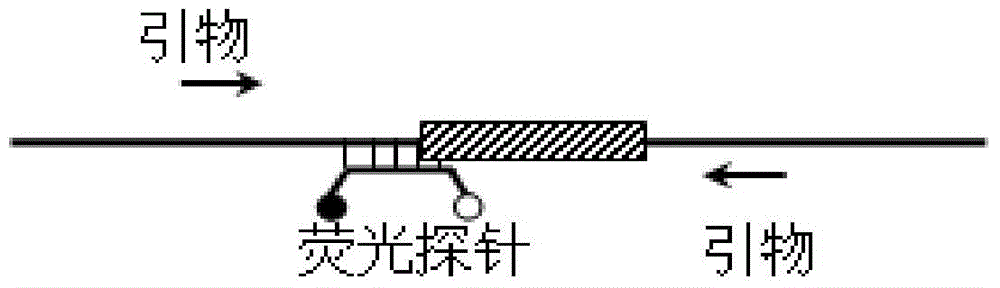
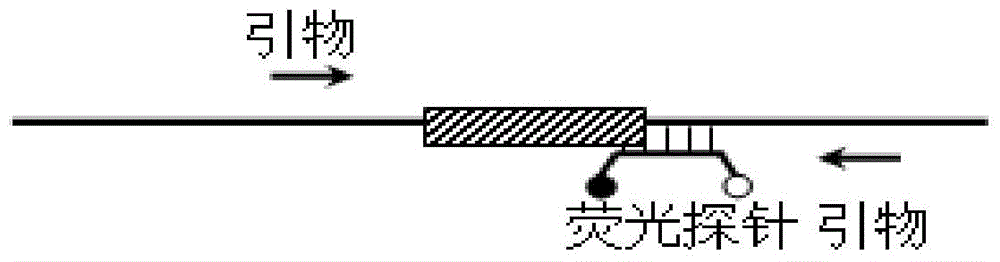
Method for detecting deletion mutation of nucleic acid molecule
InactiveCN104651529ANot easy to cause pollutionSimple and fast operationMicrobiological testing/measurementAfter treatmentReaction system
The invention relates to a method for detecting deletion mutation of a nucleic acid molecule, and particularly relates to a method for detecting deletion mutation of a nucleic acid molecule through a probe melting curve, and a reaction system and kit for detecting deletion mutation of a nucleic acid molecule. The method provided by the invention is accurate and stable, rapid, simple and convenient, free of PCR after-treatment and capable of overcoming defects that the operation is complex, long time is consumed, pollution is easily caused, the detecting flux cannot meet the conventional demand and the like in the prior art, so that the method has a favorable application prospect.
Owner:XIAMEN UNIV
Membrane virus host range mutations and their uses as vaccine substrates
InactiveUS7335363B2SsRNA viruses negative-senseSsRNA viruses positive-senseViral VaccineEngineered genetic
The present invention is directed to genetically engineered, membrane-enveloped viruses with deletion mutations in the protein transmembrane domains. Also provided are viral vaccines based on the engineered viruses, methods of producing and using such vaccines.
Owner:RES DEVMENT FOUND
Genetic engineering bacterium for producing monophosphoryl lipid A as well as construction method and application thereof
InactiveCN102399736AReduce manufacturing costConducive to large-scale industrial productionBacteriaMicroorganism based processesMicrobiologyBacterial strain
The invention discloses a genetic engineering bacterium for producing a monophosphoryl lipid A as well as a construction method and application thereof, belonging to the field of a genetic engineering. The genetic engineering bacterium is E.coli W3110 delta lacI lacZ::FnlpxE, wherein the lacI takes place a deletion mutation to lose activity and an expression FnlpxE gene is knocked into the lacZ gene. According to the bacterial strain constructed in the invention, on the basis of keeping a lipid structure single, the production cost is also reduced; no exogenous resistance genes are introduced into the bacterial strain so that the industrial production of the bacterial strain is easier to realize in a large scale.
Owner:JIANGNAN UNIV
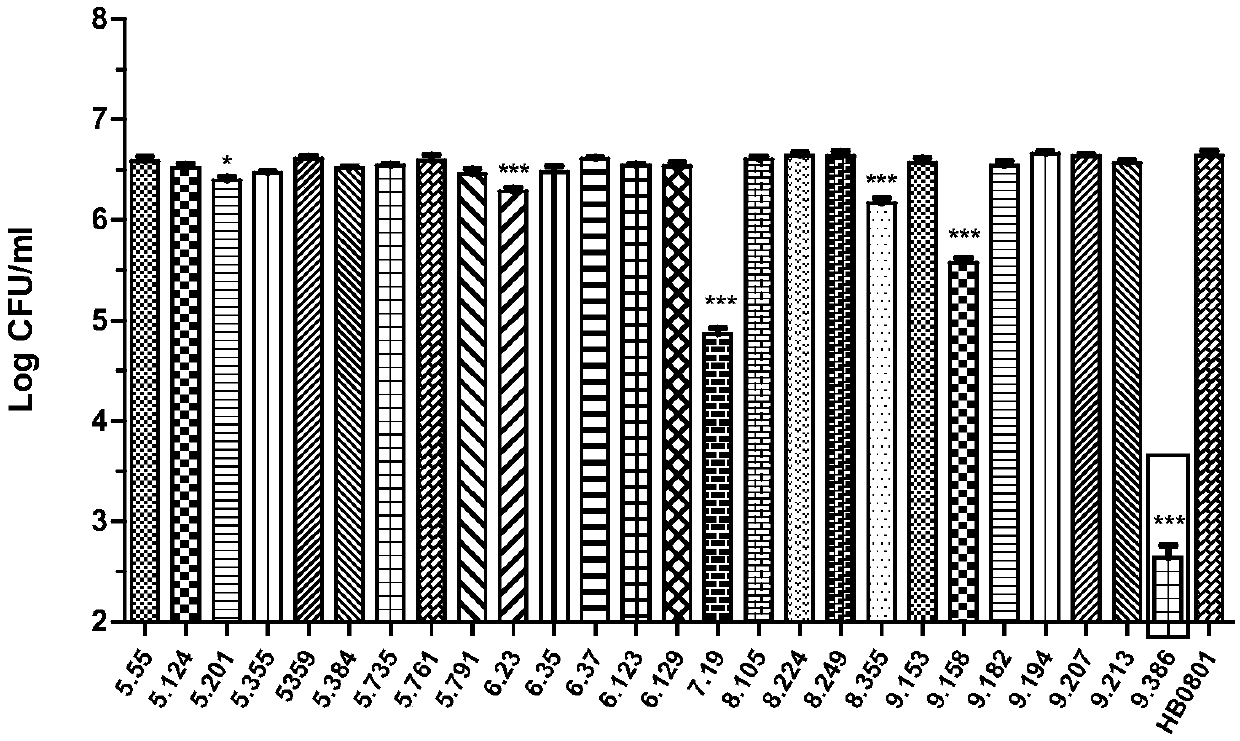
Mycoplasma bovis mutant strain with growth defect under cell coculture and application
ActiveCN109652357AMarked growth defectSignificantly small colony phenotypeBacteriaHydrolasesPhosphodiesteraseBovine embryo
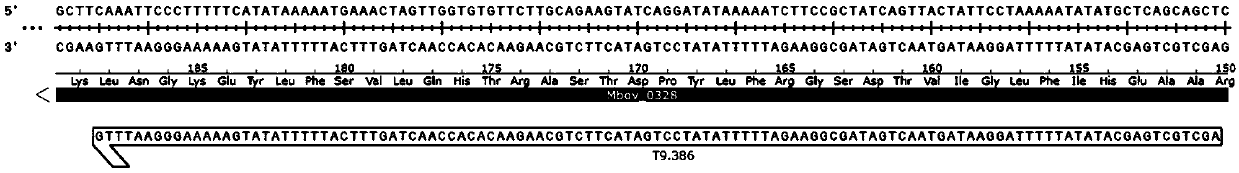
The invention belongs to the technical field of zoonosis prevention and treatment, and particularly relates to a mycoplasma bovis mutant strain with a growth defect under cell coculture and application. The mycoplasma bovis Mbov-0328 gene deletion mutant strain T9.386 is sifted out from a mycoplasma bovis gene mutant library, and the gene is coded to form cyclic dinucleotide phosphodiesterase. When the mutant strain and a bovine embryo pneumonocyte are co-cultured, the remarkable growth defect phenotype is shown; the mutant strain shows small colonial morphology on a PPLO solid culture medium.Proteomics between the mutant strain and a wild strain shows a remarkable difference expression spectrum. The mutant strain has 38 differential expression proteins, wherein 30 proteins are in up-regulated expression, and 8 proteins are in down-regulated expression. The mutant strain can be applied to the field of mycoplasma bovis metabolic physiology, pathopoiesia and immune prevention.
Owner:HUAZHONG AGRI UNIV
Fluorescence quantitative PCR detection kit for beta-thalassemia mutation
InactiveCN102851366AHigh detection sensitivityStrong specificityMicrobiological testing/measurementPositive controlBeta thalassemia
The invention relates to a fluorescence quantitative PCR detection kit for beta-thalassemia mutation. The kit comprises a PCR mixing reaction liquid, a positive control and fluorescence probes for detecting beta-thalassemia mutation genotype, wherein the PCR mixing reaction liquid contains PCR primers for amplifying a gene fragment on which a mutation site is positioned, and the mutation site is at least one mutation site selected from deletion mutation of a base corresponding to the site 41 / 42 amino acid of beta-globin gene, C-to-T mutation of a base corresponding to the site 654 amino acid of the second intron of beta-globin gene, A-to-T mutation of a base corresponding to the site 17 amino acid of beta-globin gene, A-to-G mutation of a base corresponding to the site 28 amino acid on the upstream of the promoter of beta-globin gene, A base insertion mutation of a base corresponding to the site 71 / 72 amino acid of beta-globin gene, and G-to-C mutation of a base corresponding to the site 5 amino acid of the first intron of beta-globin gene. With the technical scheme of the present invention, rapid, accurate and high sensitivity detection of mutation conditions of beta-thalassemia gene can be achieved, and especially 6 special site mutations of beta-thalassemia gene can be detected, wherein the 6 special site mutations are common in Chinese.
Owner:广州达健生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com