Human profibrinolysin kringle 5 deletion mutation recombinant polypeptide
A human plasminogen, recombinant polypeptide technology, applied in the direction of recombinant DNA technology, enzymes, enzymes, etc., can solve the problems of vascular leakage without satisfactory treatment methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
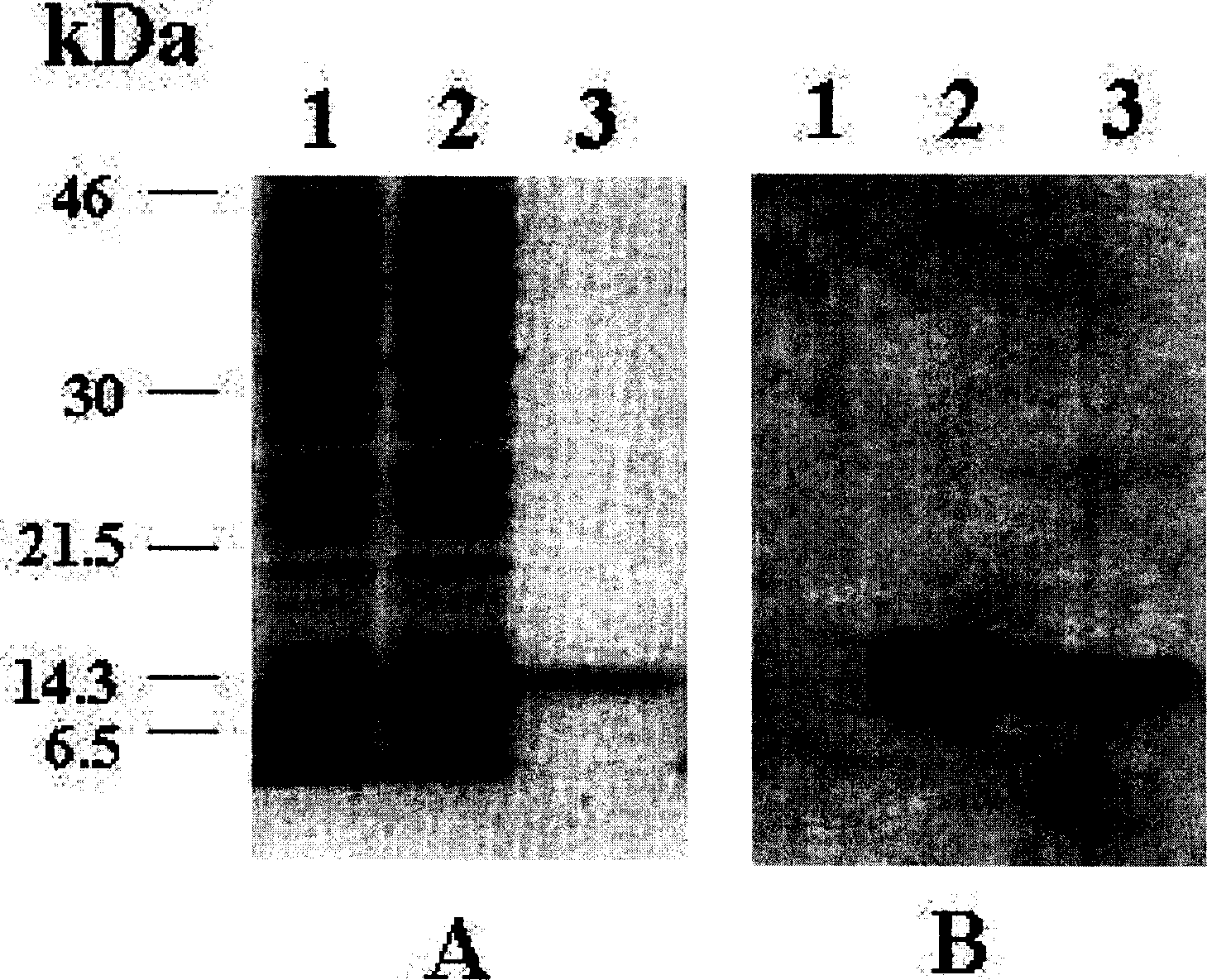
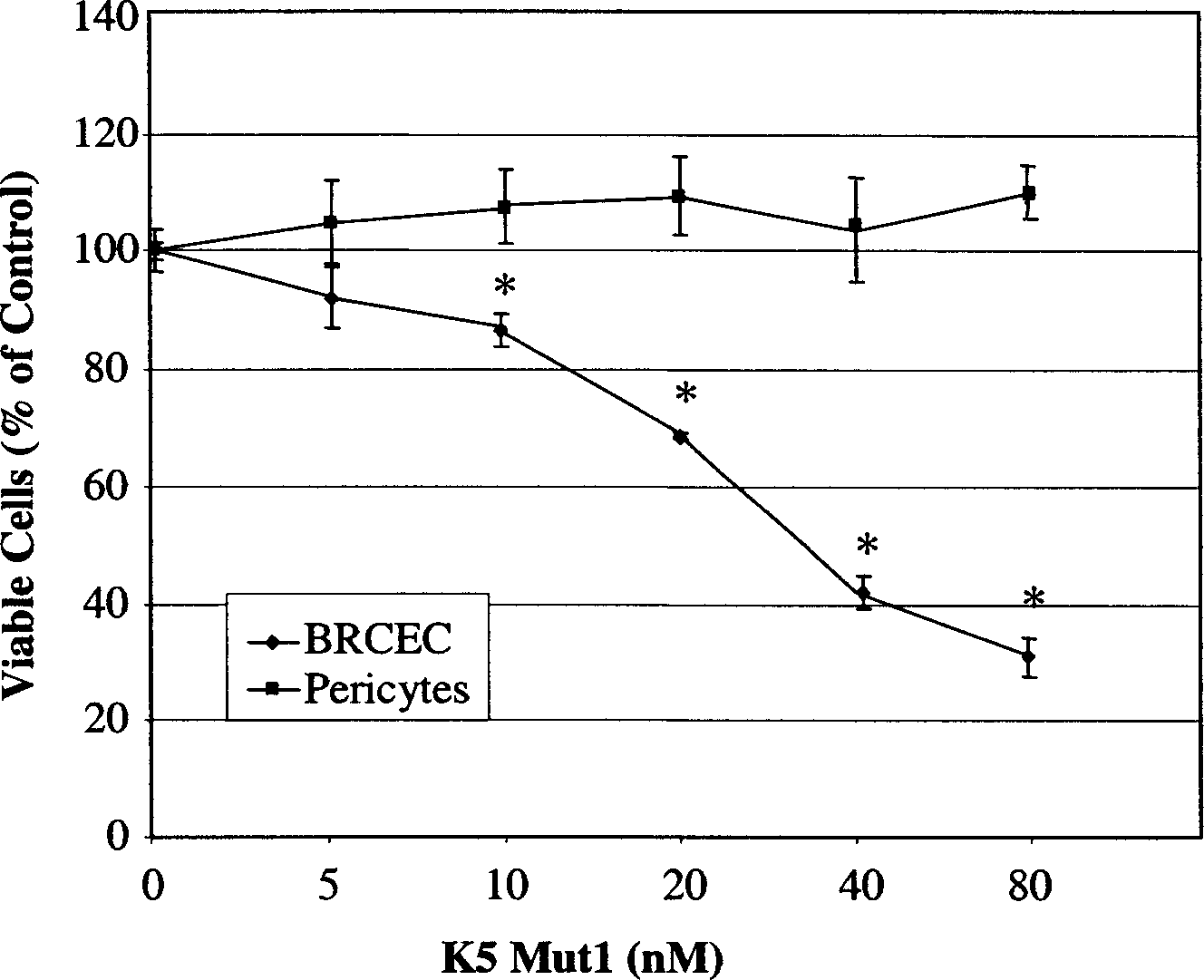
[0016] Materials: Retinal capillary endothelial cells (RCEC) and epithelial cells were isolated from bovine eyes as described previously (Grant, M.B. et al. Invest Ophthalmol. Vis. Sci., 32:53-64, 1991). It is shaped like a pebble, bound to fluorescent probe DiI-labeled acetylated low-density lipoprotein (Biomedical Technologies Inc., Stoughton, MA), confirmed as RCEC, cultured epithelial cell purity by α-smooth muscle actin-specific FITC binding Antibody identification. Brown-Norway rats were purchased from Harlan (Indianapolis, IN).
[0017] 1. Construction of K5mut1:
[0018]Human K5 cDNA was amplified from liver RNA by RT-PCR (Ma J.X. et al. FEBS Lett, 452:199-204, 1999). The deletion mutant was amplified by PCR using the human K5 template. The 5'PCR primer (5'-ATGAATTCGTGTATGTTTGGGAATGGG-3') and 3'PCR primer (5'-GCCAAGCTTACACTGAGGGACATCACAGTAG-3') each contained EcoR I and HindIII sites to facilitate cloning. PCR deleted the nucleotide sequence encoding 10 amino acid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com