Bacterial laccase mutant protein, recombinant expression plasmid, transformed engineered strain and fermentation preparation method thereof
A technology of mutants and engineering bacteria, which is applied in the field of enzyme genetic engineering technology and fermentation engineering, can solve the problems that hinder the industrial application process of bacterial laccase, and achieve the effect of facilitating large-scale industrial production and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1, utilize PCR method to carry out the construction of deletion mutant DNA coding sequence
[0026] A total of 10 amino acids (323GAMSRRMMQG332) upstream of the protein sequence including the glycine at position 332 of the break site were selected for site-directed deletion. Primers were designed as follows:
[0027]
[0028]
[0029]Using the expression vector pET-22b(+)-lac15 containing the coding sequence of the wild-type bacterial laccase Lac15 gene as a template, the first round of PCR was carried out with lac15_F and the deletion primer D(323-332)_R, and at the same time, lac15_R and the mutant primer D were used (323-332)_F performed the second round of PCR. The first-round and second-round PCR products were recovered as templates for the third round of PCR, and lac15_F and lac15_R were used for the third round of PCR to obtain the full-length sequence with deletion mutations. The obtained deletion mutant gene sequence was connected to pET22b...
Embodiment 2
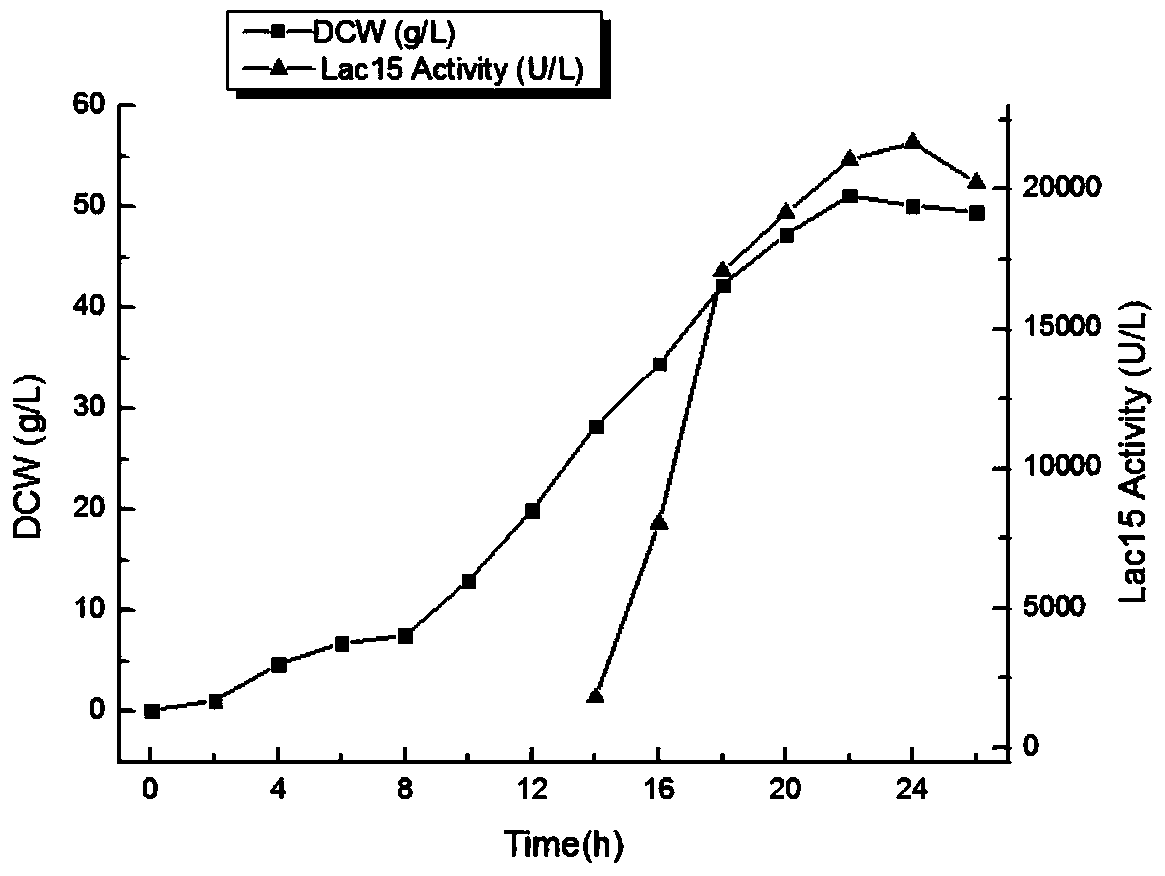
[0031] Example 2. Induced expression and purification of mutant proteins
[0032] The recombinant engineered bacteria Escherichia coli BL21(DE3) / pET-22b(+)-lac15(Del) and the wild-type gene recombinant engineered bacteria Escherichia coli BL21(DE3) / pET-22b(+)-lac15 confirmed by sequencing were inoculated into the In 5ml of liquid LB medium, the inoculum size was 1%, and cultured with shaking at 220rpm at 37°C for 8-12h. Insert the overnight cultured recombinant engineered bacterium into 400ml liquid LB medium according to the inoculum size of 1%, 37 ℃, 200rpm shaking culture, when the fermentation broth OD 600 When it reaches 0.6-1.0, add IPTG to a final concentration of 0.2mM, and at the same time lower the temperature to 28°C to induce expression for 12-14h.
[0033] Collect the cells by centrifugation at 4°C at 8000g, add 0.1 times the volume of the bacterial solution in Binding buffer, break the cells by ultrasonication for 40min in a 350W ice bath, and collect the supe...
Embodiment 3
[0035] Embodiment 3, wild-type bacterial laccase and its mutant Lac15 (Del) stability experiment
[0036] The purified wild-type bacterial laccase and its mutant proteins were stored at 28°C with a buffer of 50mM Na 2 HPO 4 -KH 2 PO 4 (pH 7.5). Regularly detect the remaining enzyme activity of wild-type bacterial laccase and its mutant protein, and at the same time, carry out SDS-PAGE detection on the samples.
[0037] Such as figure 1 As shown, the SDS-PAGE electrophoresis pattern on the 4th day, 7th day, 15th day and 22th day showed that the wild-type protein was degraded by about 80% on the 4th day, and the mutant protein was degraded by about 50% on the 14th day. The half-life of the body protein at 28°C is 3-4 times that of the wild type.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com