Production method for Fbxo40 gene knockout pigs
A gene knockout and gene technology, applied in the field of animal genetic engineering and genetic modification, can solve the problems of gene function loss, gene frameshift mutation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Construction of CRSIPR / Cas9 Targeting Vector pX3301-8
[0031] 1. Design and screening of porcine Fbxo40 gene-specific sgRNA
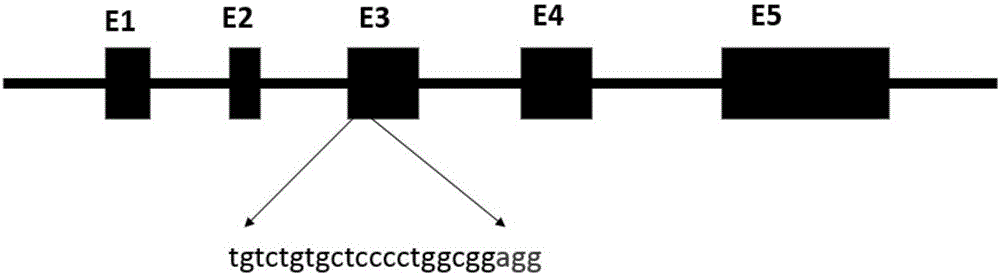
[0032] The sequences of exon 3 and exon 4 of porcine Fbxo40 gene encode important domains of Fbxo40 protein—zinc-finger domain and F-box domain.
[0033]According to the working principle of CRISPR / Cas9, a total of 17 sgRNA sequences were designed at different positions of the third exon and the fourth exon. Its sequence and complementary sequence are shown in Table 1.
[0034] Table 1
[0035]
[0036]
[0037] The bold part in Table 1 is the PAM sequence recognized by CRISPR / Cas9.
[0038] The backbone of the pX330 vector needs to be digested with BbsI, so the cohesive end of the BbsI restriction site needs to be added to the sgRNA sequence to facilitate its ligation into the backbone of the pX330 vector.
[0039] The designed sgRNA and its complementary sequence added to the cohesive end of the BbsI restriction site were s...
Embodiment 2
[0052] Example 2 Establishment of pig fetal fibroblasts
[0053] Anesthetize the 30-day-pregnant piglet, aseptically remove the fetus from the uterus, wash the fetus with PBS containing double antibodies, place it in an ultra-clean workbench, and remove the head, limbs, internal organs and cartilage of the fetus with ophthalmic scissors Rinse the tissue with PBS; use ophthalmic scissors in the cell culture dish to cut the remaining tissue into about 1mm 3 Small pieces; add an appropriate amount of FBS to keep the tissue from drying out too much. Transfer the shredded tissue pieces to a T75 cell culture flask, and spread the tissue pieces evenly; add 5 mL of cell culture medium, with the side covered with the tissue pieces facing up, without being submerged by the culture medium, store at 37°C, 5% CO 2 After culturing in the incubator for 3 to 5 hours, turn the T75 over so that the tissue block is submerged in the culture medium; after culturing for about 3 days, it is observ...
Embodiment 3
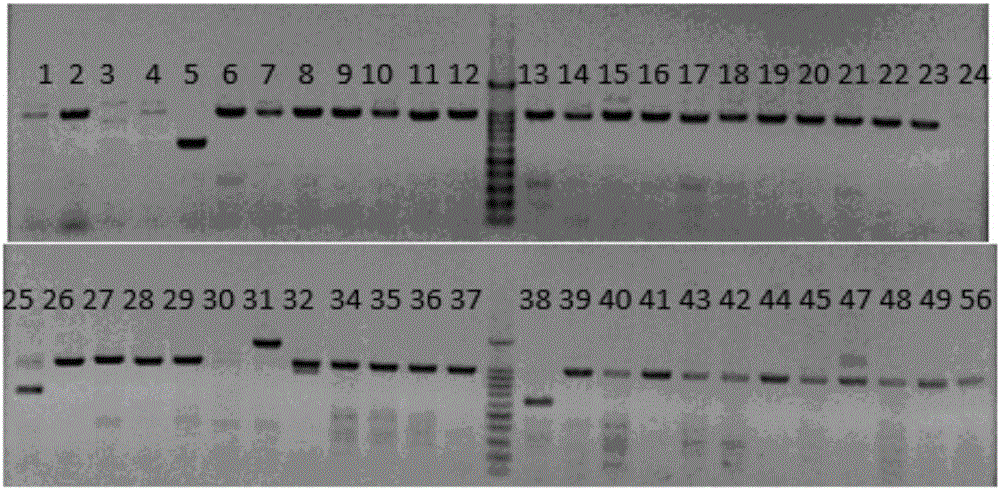
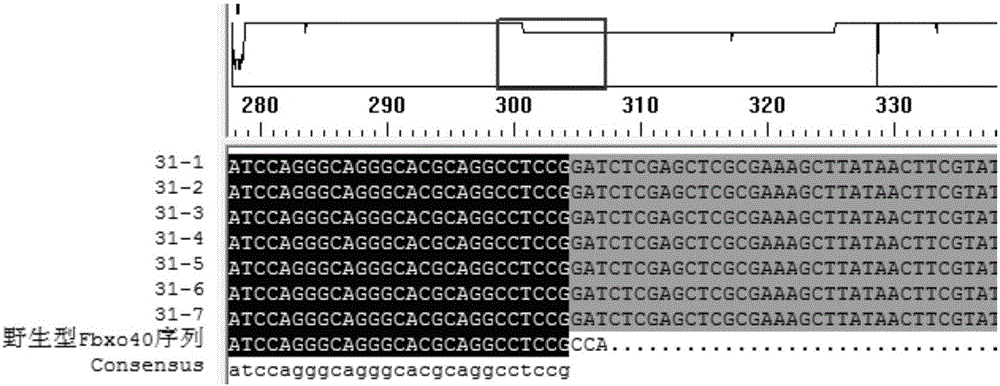
[0054] Example 3 Transfection of pig fetal fibroblasts and screening of target cell monoclonal
[0055] 1. Digestion of PGK-Neo plasmid
[0056] The PGK-Neo plasmid was digested with restriction endonucleases NotI and NheI from NEB Company, and kept in a water bath at 37°C for 4h. The PGK-Neo fragment with a size of 1.8 kb was recovered using Genstar's Gel Extraction Kit. After the concentration was measured, it was subpackaged and frozen in a -20°C refrigerator for later use.
[0057] 2. Digest and centrifuge porcine fetal fibroblasts that have reached 80-90% confluence in a six-well plate, and obtain about 2×10 5 -2×10 6 porcine fetal fibroblasts.
[0058] 3. Add 2 μg of the CRISPR / Cas9 targeting vector pX3301-8, and add it to the Lonza transfection reagent at a molar ratio of 3:1 with the PGK-Neo gene linear vector, and mix well. The cells were resuspended using the transfection reagent added with the plasmid, and the cell suspension was added to the electric shock cup...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com