Mutated cephalosporin C acylase
A cephalosporin, acylase technology, applied in bacteria, hydrolase, biochemical equipment and methods, etc., can solve the problems of low substrate conversion rate and low enzyme activity, and achieve high expression activity, efficient catalysis, good The effect of industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Gene Mutation of Mutant Cephalosporin C Acylase CPCacy-D2
[0026] Starting from the gene sequence corresponding to the original CPC acylase SEQ ID NO: 1 (SEQ ID NO: 4 described in Chinese invention patent ZL 200810102219.7), using pUC18-acy (Chinese invention patent ZL 200810102219.7) as a template, using polymerization Construction of Mutant Cephalosporin C Acylase CPCacy by Enzyme Chain Reaction (PCR) Method M . According to the steps described in the TaKaRa MutanBEST kit, a pair of Up1-Down1 primers (Up1: GGCGGTGATGCCAGCGACGCA; Down1: TCCAGCCGTTGATGCATTACTGAAA) were used to reversely amplify the plasmid pUC18-acy to delete 227-228 at the C-terminus of the α subunit The two amino acid residues of Ala-Met.
[0027] The reaction system for PCR is: sterile water 37.7 μL, 10 × Pyrobest Buffer, 5 μL; dNTPs (each dNTP concentration 2.5 mM), 4 μL; upstream and downstream primers (concentration 20 μmolL -1 ), 1 μL each; plasmid template, 1 μL; Pyrobest DNA polymerase (5 U...
Embodiment 2
[0031] Mutation of the improved mutant cephalosporin C acylase CPCacy-D4 gene and construction of its transformant
[0032]Other conditions are the same as in Example 1, but the PCR primers used are changed to Up2-Down2, wherein the gene sequence of Up2 is: GCATTACGTCCAGCCGTTGATGCAT; the gene sequence of Down2 is: AGGCGTGGAAGCGGAGCGTCTGGAG. The recombinant plasmid pUC18-CPCacy was obtained after PCR amplification and ligation M-D4 , a mutant CPC acylase gene carrying a deletion at positions 212-215 of Ala-Asp-Leu-Ala. The amino acid sequence of the encoded protein is SEQ ID NO: 3, which is the mutant CPCAcy-D4.
Embodiment 3
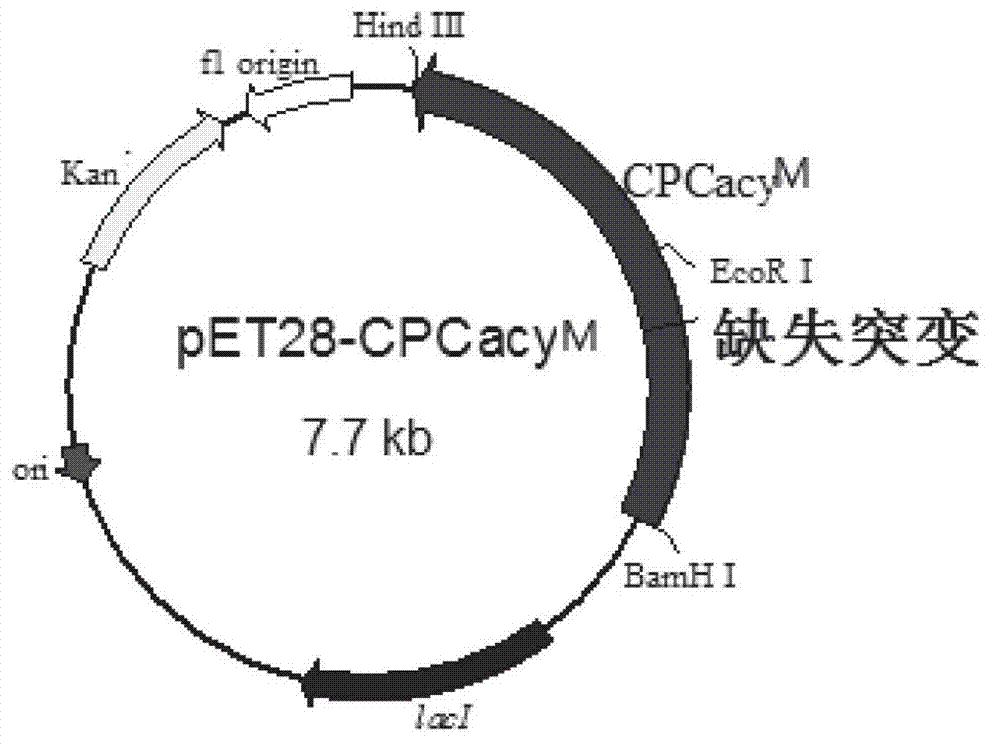
[0034] Expression vector pET28-CPCacy M and pMKC-CPCacy M Construction and transformant construction
[0035] (1) Plasmid pET28-CPCacy M-D2 Construction: the plasmid vector pUC18-acy obtained in Example 1 M-D2 and pET28a (Novagen Company) were cut with BamHI / HindIII double enzymes respectively. The volume of enzyme digestion reaction is 50 μL, the amount of plasmid is 17 μL, the amount of each enzyme is 1 μL, buffer solution is 5 μL, and it is made up to 50 μL with sterile water. React overnight at 37°C to obtain the restriction product CPC acylase gene and the linear plasmid backbone of pET28a. After the PCR product recovery kit (Tiangen Biochemical Technology (Beijing) Co., Ltd.) was used to purify the digested product, the CPC acylase gene and the linearized plasmid backbone of pET28a were digested with T4 DNA ligase at a concentration ratio of 3:1 at 4°C. Ligation reaction, the connection reaction time is 14 h to 16 h. Transform the ligation reaction into the host s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com