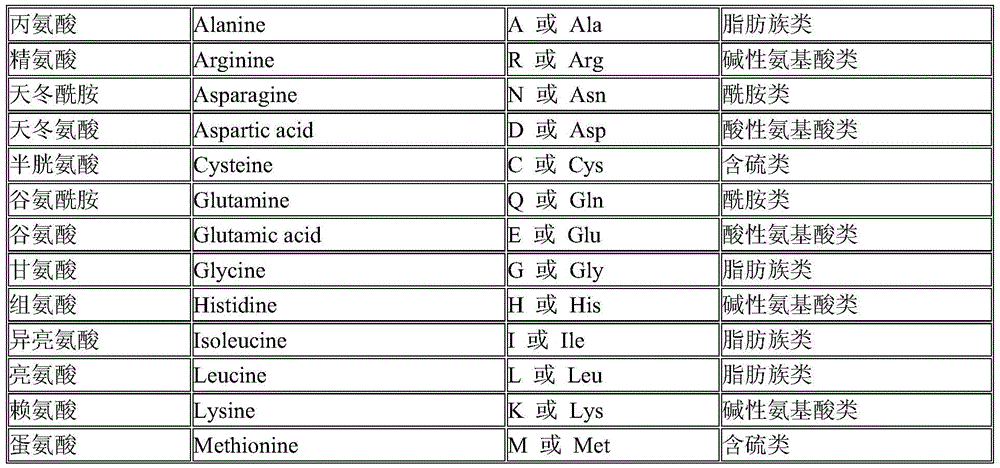
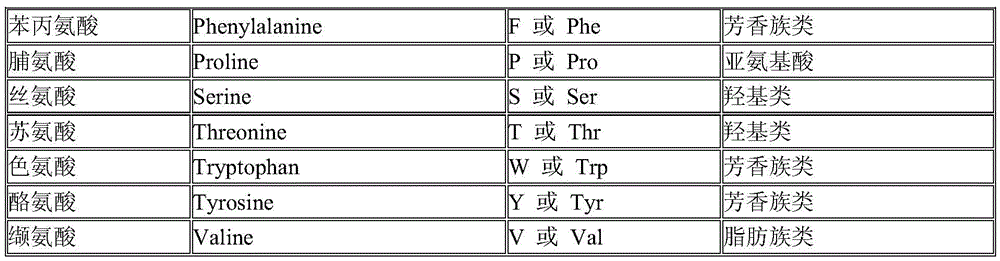
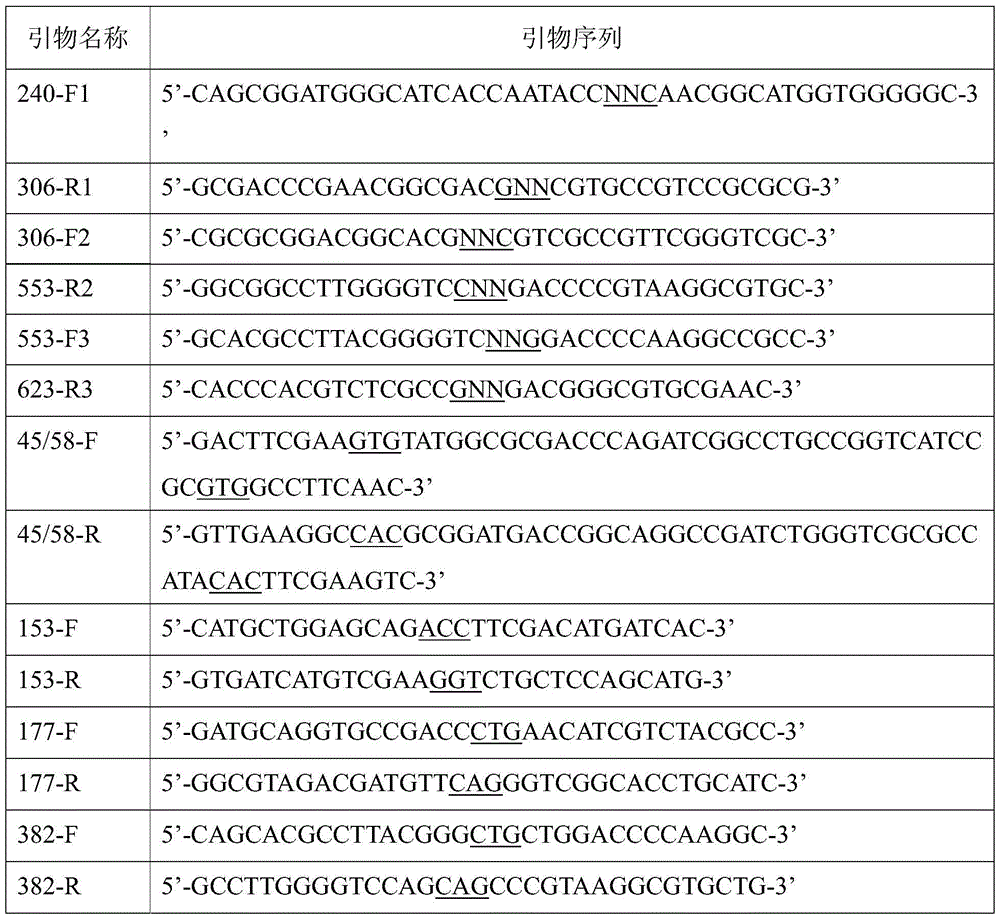
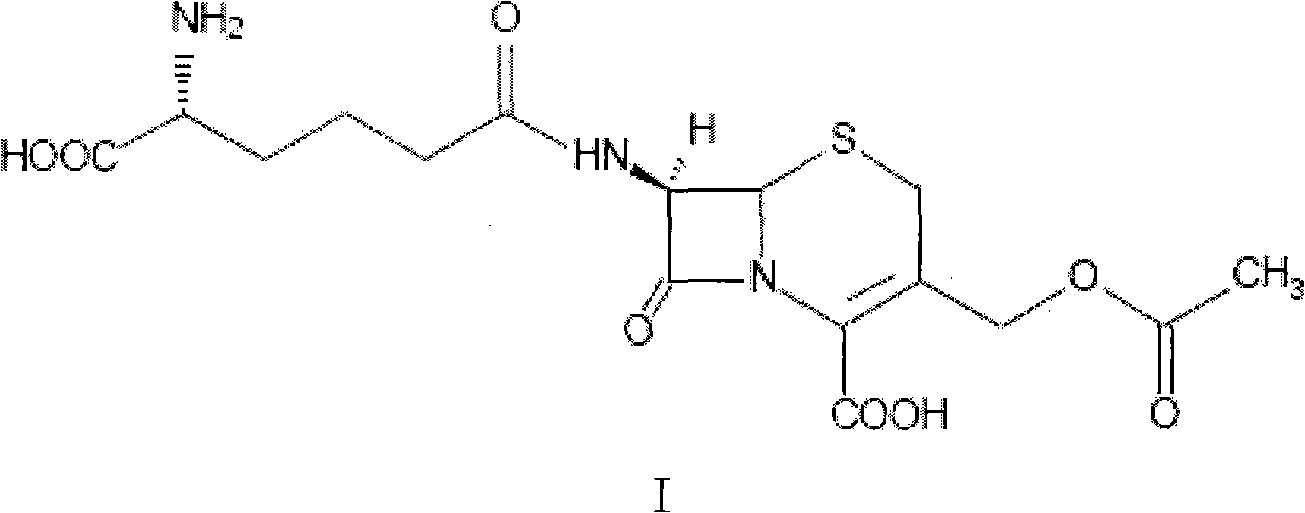
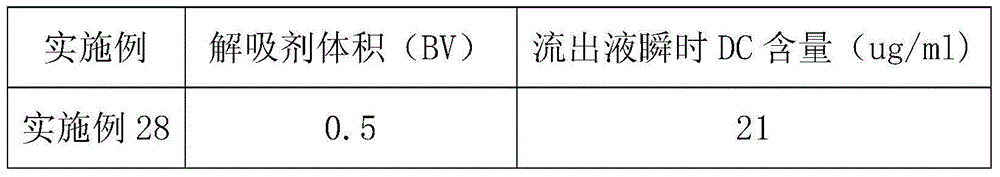
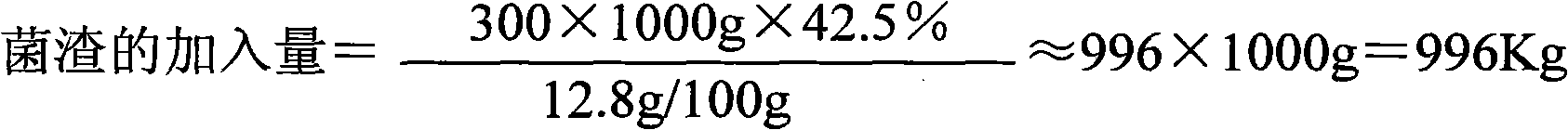Patents
Literature
103 results about "Cephalosporin C" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
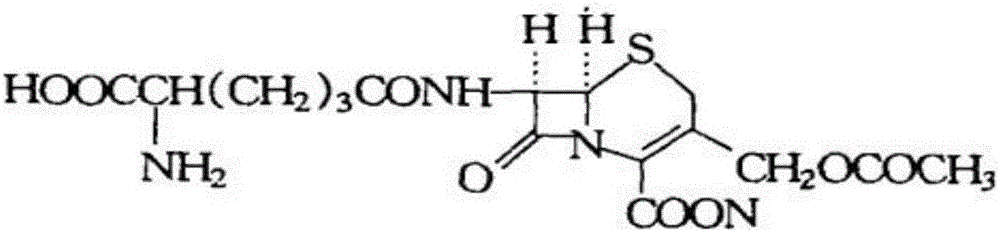
Cephalosporin C is an antibiotic of the cephalosporin class. It was isolated from fungi of the genus Acremonium and first characterized in 1961. Although not a very active antibiotic itself, synthetic analogs of cephalosporin C, such as cefalotin, became some of the first marketed cephalosporin antibiotic drugs.
Preparation method of immobilized cephalosporin C acylase
The invention discloses a preparation method of immobilized cephalosporin C acylase and belongs to the field of biotechnology. According to the preparation method disclosed by the invention, cephalosporin C acylase is immobilized on a carrier in the presence of acetate and / or bicarbonate to obtain the immobilized cephalosporin C acylase. The immobilized cephalosporin C acylase which is prepared by using the preparation method disclosed by the invention has high activity and thermal stability, and the preparation method disclosed by the invention is simple, stable in process and low in production cost.
Owner:UNIV OF SCI & TECH BEIJING
Cephalosporin C acylase mutant
The invention relates to a cephalosporin C acylase mutant. Cephalosporin C acylase is constructed through a point mutation method, in comparison with wild type cephalosporin C acylase coming from Pseudomonas sp.GK16, the activity of the cephalosporin C acylase is improved by 20.5-150 times, and the cephalosporin C acylase mutant can be used for producing 7-ACA through a one-step enzymatic method.
Owner:上海邦林生物科技有限公司
Cephalosporin C acylase mutant as well as coding gene and application thereof
The invention relates to a cephalosporin C acylase mutant as well as a coding gene and the application thereof. An amino acid sequence is shown as SEQ ID NO. 4; or after one or more amino acids are replaced, deleted or added, the sequence forms an amino acid sequence having an equivalent function and derived from the SEQ ID NO.4. The cephalosporin C acylase mutant provided by the invention has the advantages as follows: in the enzymatic reaction process of converting CPC into 7-ACA by using the mutant, the conversion rate reaches 99% and the yield reaches 96%.
Owner:ANHUI BBCA GENETIC ENG TECH CO LTD
Macroporous adsorption resin special for extracting cephalosporin C and its preparation method
The invention relates to a macro-porous adsorption resin specially used for extracting cephalosporin C and a preparation method for the macro-porous adsorption resin. The specific surface of the adsorption resin is ranging from 1000 to 2000m<2> / g and the pore volume thereof is 1.5-2.5ml / g. The preparation method is that dispersant is firstly dissolved in dispersion medium and mixture consisting of monomer, pore-forming agent and photopolymerization initiator are also added in the dispersion medium, so as to carry out the reaction for 5-10 hours under the suspension polymerization temperature of 60-100 DEG C and to gain the crosslinking macro-porous copolymer substrate; the macro-porous copolymer substrate is disposed by Lewis acid catalyst under the conditions of existence of inert medium and the temperature of 50-120 DEG C and the crosslinking reaction is carried out again inside the substrate. The macro-porous adsorption resin specially used for extracting cephalosporin C of the invention has high adsorption rate, good selection to the object output, easy resolution and regeneration and high purity of the extracted cephalosporin C products.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
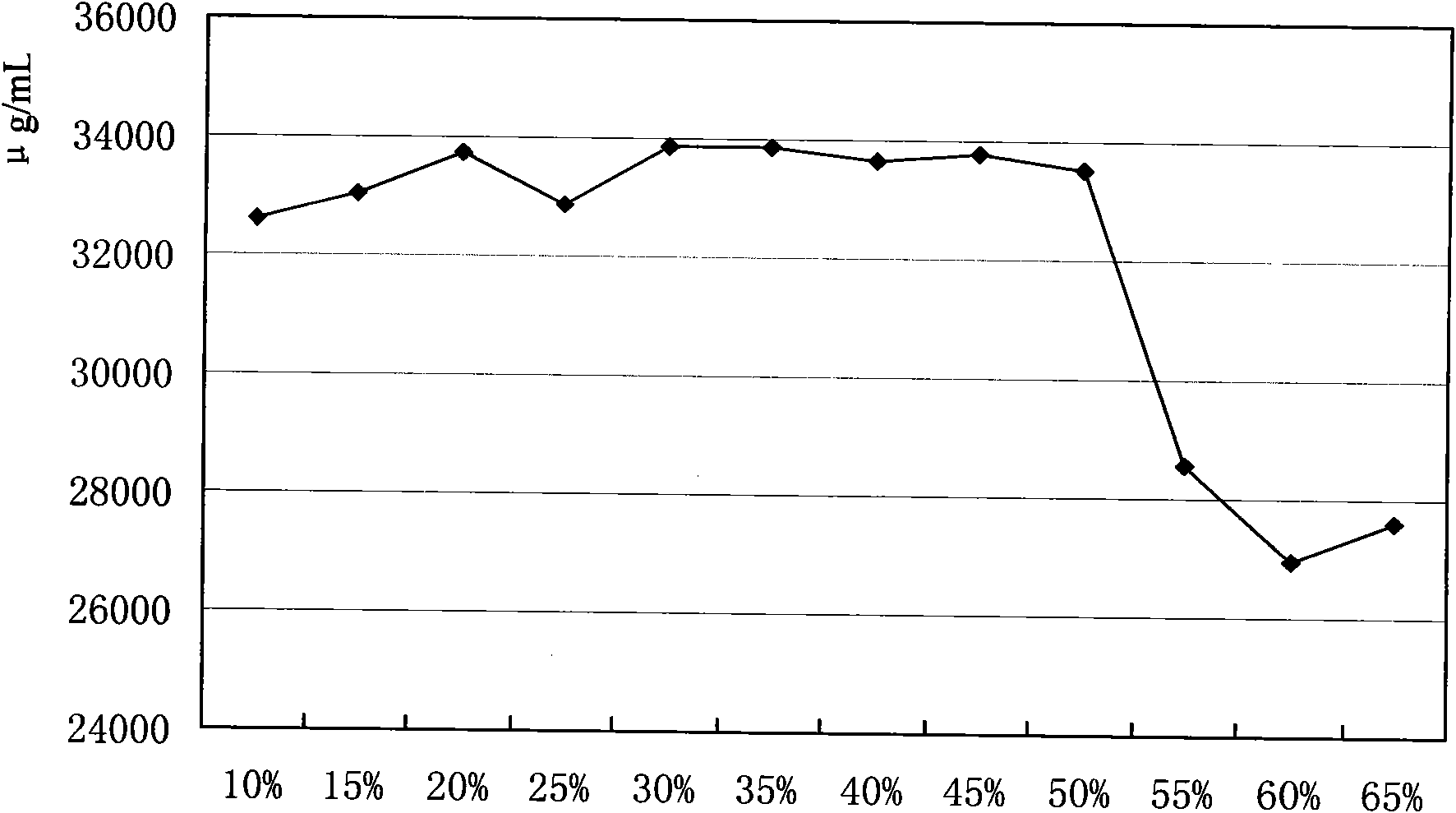
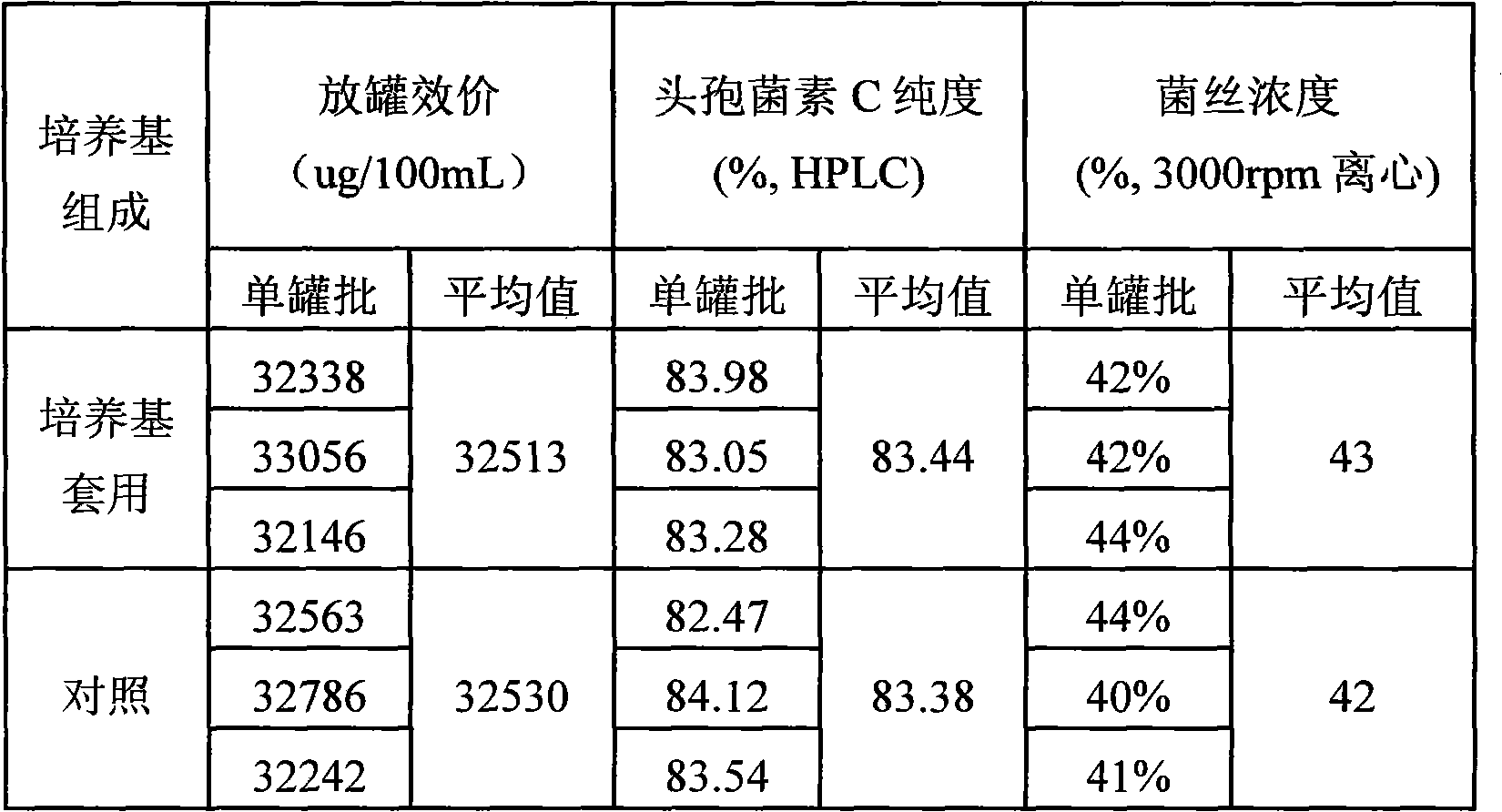
Application method for cephalosporin C bacterium residue
InactiveCN101935682AReduce manufacturing costReduce the amount addedChemical recyclingFermentationNitrogen sourceCarbon source
The invention relates to an application method for cephalosporin C bacterium residue, which uses the cephalosporin C bacterium residue as nutrition constituent of cephalosporin fermentation medium, and comprises the following steps: (1) collecting bacterium residue and measuring biochemical indicator thereof; (2) selecting replaced substance; (3) measuring the content of protein of the replaced substance, namely peanut cake powder; (4) determining application proportion; (5) calculating the addition of the bacterium residue; and (6) when blending the base materials of the cephalosporin fermentation medium, reducing the usage content of the peanut cake powder and artificial gum and adding the bacterium residue of calculated content according to the calculated result. The invention belongs to the technical field of biomedicine, uses the circulating application of the cephalosporin C bacterium residue to reduce the addition content of the nitrogen source peanut cake powder and carbon source artificial gum nutrition constituent raw materials, thereby reducing the production cost of the cephalosporin C and the discharge of the abandoned bacterium residue, decreasing the environmental pollution problem caused by abandoned bacterium residue, and being beneficial to realize cleaner production. And the invention has simple and convenient operation.
Owner:石药集团中诺药业(石家庄)有限公司
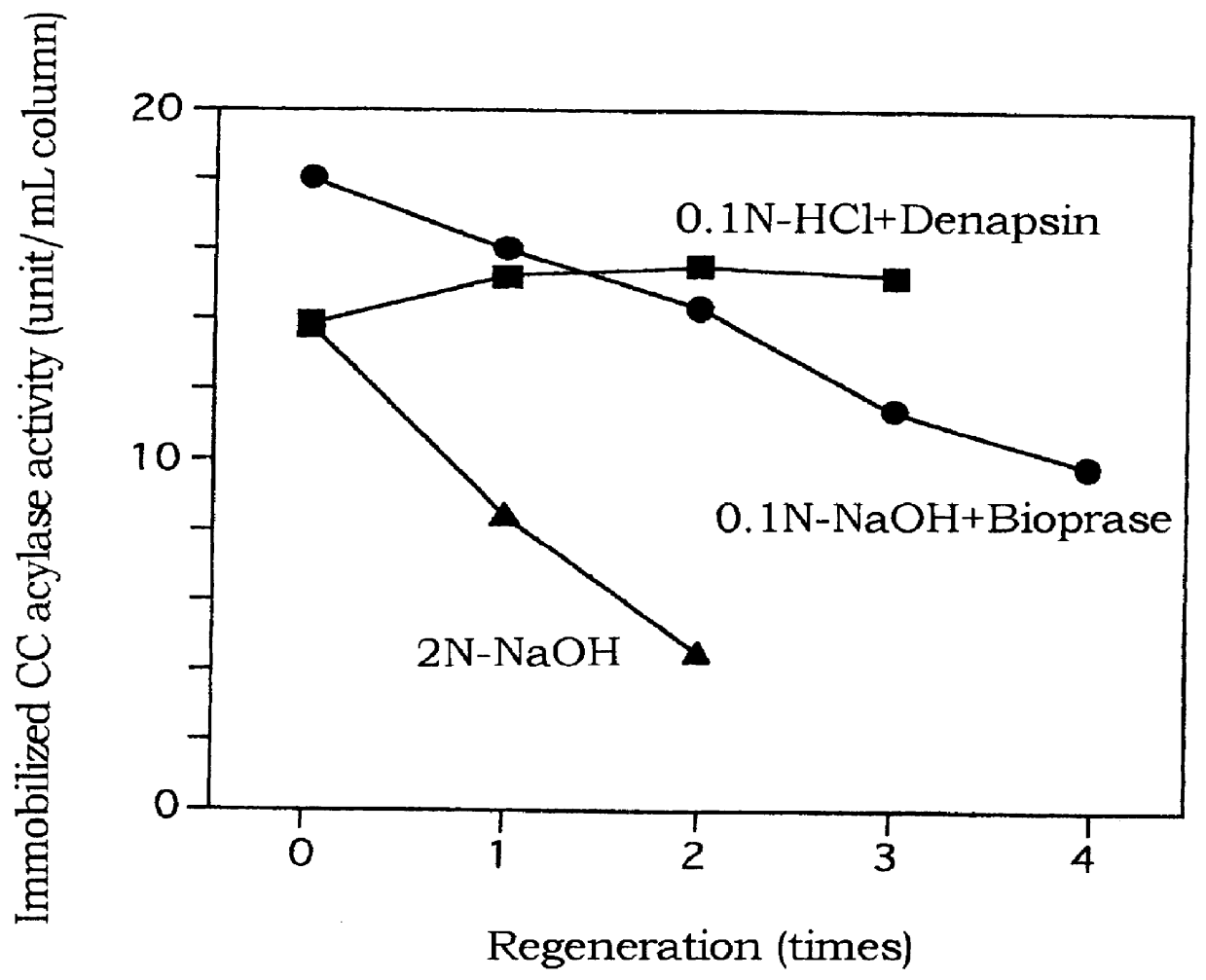
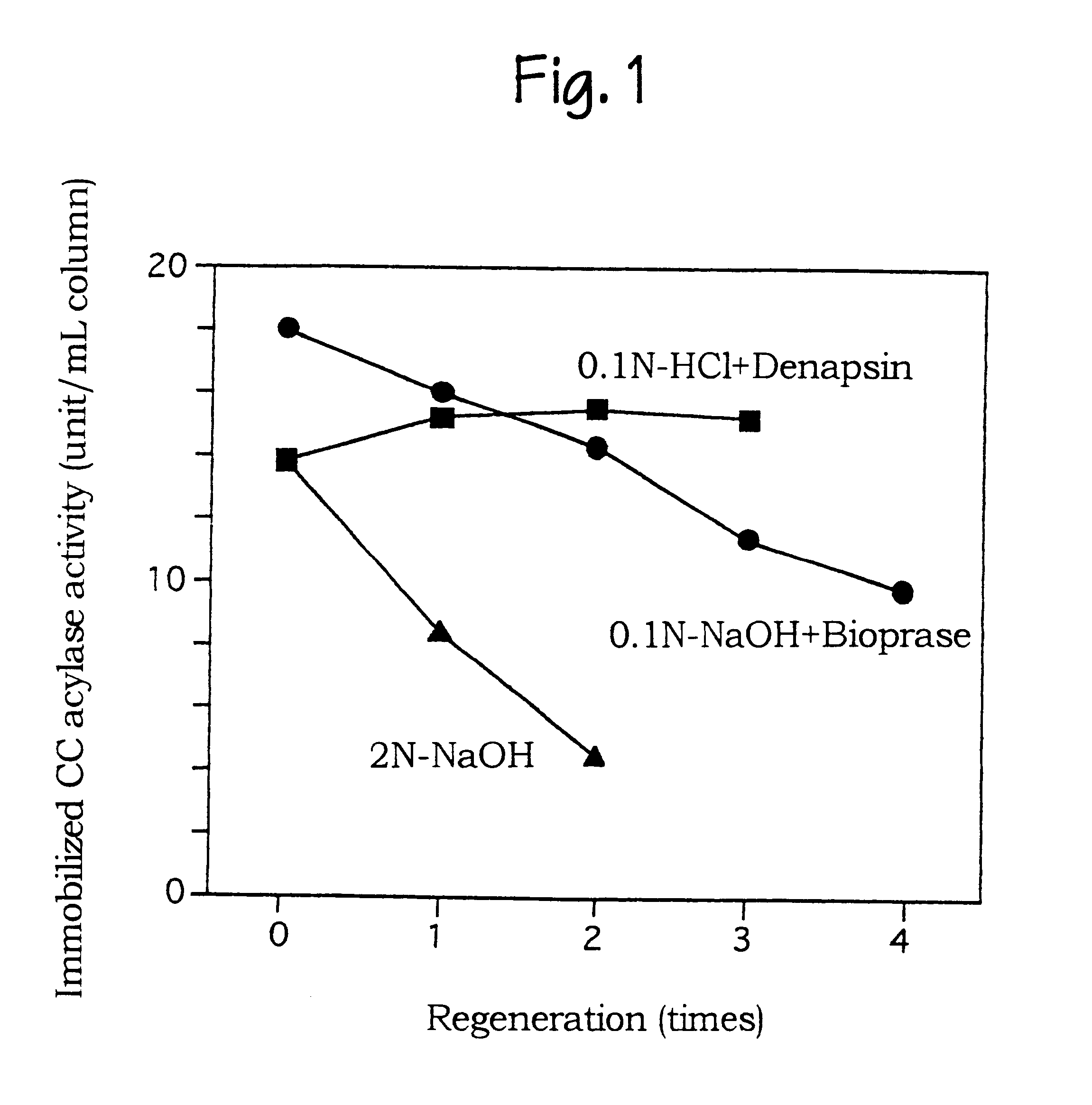
Purifying cephalosporin C acylase and regenerating a carrier immobilizing cephalosporin C acylase
InactiveUS6165758AHigh purityHigh productHydrolasesOn/in organic carrierProteinase activitySURFACTANT BLEND
PCT No. PCT / JP97 / 02663 Sec. 371 Date Feb. 12, 1999 Sec. 102(e) Date Feb. 12, 1999 PCT Filed Jul. 30, 1997 PCT Pub. No. WO98 / 06829 PCT Pub. Date Feb. 19, 1998An enzyme in a mixture containing the enzyme and a contaminant enzyme is purified by selectively aggregating and precipitating the contaminant enzyme with a surfactant. A carrier containing an immobilized enzyme is regenerated by using a protease to remove the enzyme from the carrier. Cephalosporin C acylase is purified from a mixture of the acylase and a deacetylase contaminant by selectively aggregating and precipitating the deacetylase contaminant by adding to the solution a benzylated cationic or methylated cationic surfactant in an amount of 0.1 to 0.6% of the mixture. The surfactant may be alkyl(palm)dimethylbenzyl ammonium chloride. A carrier containing immobilized cephalosporin C acylase is regenerated by contacting the carrier with an alkaline or acidic protease. The acylase is immobilized by being bound to the carrier and optionally crosslinked. The carrier may have pores of 100 nm or less in diameter.
Owner:FUJISAWA PHARMA CO LTD
Mutant cephalosporin C acylase, method for preparing same and method for converting 7-aminocephalosporin acid (ACA)
ActiveCN102978192AImprove stabilitySmall steric hindranceHydrolasesFermentationAmino acid substitutionMutant
The invention provides a mutant cephalosporin C acylase. One or a plurality of amino acid locus replacement is carried out on an amino acid sequence shown as SEQ ID NO.1 in a sequence table to obtain a mutant amino acid sequence; and one or more of 288-locus valine, 296-locus histidine and 417-locus histidine is or are adopted as amino acid locus(es). The technical problems that the mutant cephalosporin C acylase of the prior art has small catalytic activity, seriously influenced enzymatic activity by the outside and product inhibition are solved.
Owner:HUNAN FLAG BIOTECHNOLOGY CO LTD
Resin with specific adsorption for cephalosporin C and preparation method therefor
ActiveCN104844744AImprove stabilityStrong anti-pollutionIon-exchange process apparatusOther chemical processesCross-linkAlkyl transfer
The invention discloses resin with specific adsorption for cephalosporin C and a preparation method therefor. The adsorption resin is prepared by adopting suspension polymerization. The preparation method comprises the steps of: after mixing a monomer, a cross-linking agent, a pore-foaming agent, an initiator and water, performing a suspension polymerization reaction under the condition of the existence of a conventional dispersing agent, wherein the suspension polymerization temperature is 75 to 85 DEG C and the polymerization reaction time is 10 to 16 hours; carrying out distillation and drying treatment on the obtained polymer; adding the dried polymer and an inert solvent into a reactor together for swelling, adding lewis acid at room temperature, dropwise adding an alkylation reagent, heating to 40 to 50 DEG C to perform a friedel-crafts alkylation reaction for 12 to 16 hours, and carrying out solvent washing and washing to obtain the cephalosporin C specific adsorption resin. The cephalosporin C specific adsorption resin prepared by the method has specific adsorption for cephalosporin C, and is large in adsorption quantity and high in purity.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
Cephalosporin G producing recombinant strain, construction method and applications thereof
The present invention discloses a cephalosporin G producing recombinant strain, a construction method and applications thereof. The construction method comprises: transforming an expandase gene into recipient bacteria to obtain the cephalosporin G producing recombinant strain, wherein the recipient bacteria are mutation type escherichia coli or wild-type escherichia coli. Experiment results show that the yield of the cephalosporin G producing recombinant strain is 2.67-29.01 mM (0.89-9.64 g / L).
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Technology for preparing medicine intermediate D-7-ACA by two enzyme carriers one-step method
The invention discloses a technology for preparing a medicine intermediate D-7-ACA by a two enzyme carriers one-step method, a cephalosporin C sodium salt concentrate is taken as a substrate, immobilization CPC acylase and immobilization deacetylase are employed, two enzyme carriers are mixed according to certain proportion so that a cephalosporin C extract is directly conversed to D-7-ACA. The technology for preparing the medicine intermediate D-7-ACA is capable of simplifying the technology route, raising the utilization rate of the cephalosporin C fermentation components, shortening the production period, avoiding the usage of liquid oxygen in a three enzyme carriers two-step method and enhancing the production security, the whole technology employs the enzyme method production technology and has the advantages of mild condition, simple equipment, no pollution, high yield, less by-product, low cost and the like, and is in favor of development of the national green pharmacy industry and is a sustainable technology route.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
Cephalosporin C acylase mutant with higher heat stability and construction method thereof
ActiveCN106676090AImprove stabilityLow degree of inactivationHydrolasesVector-based foreign material introductionProtein targetHalf-life
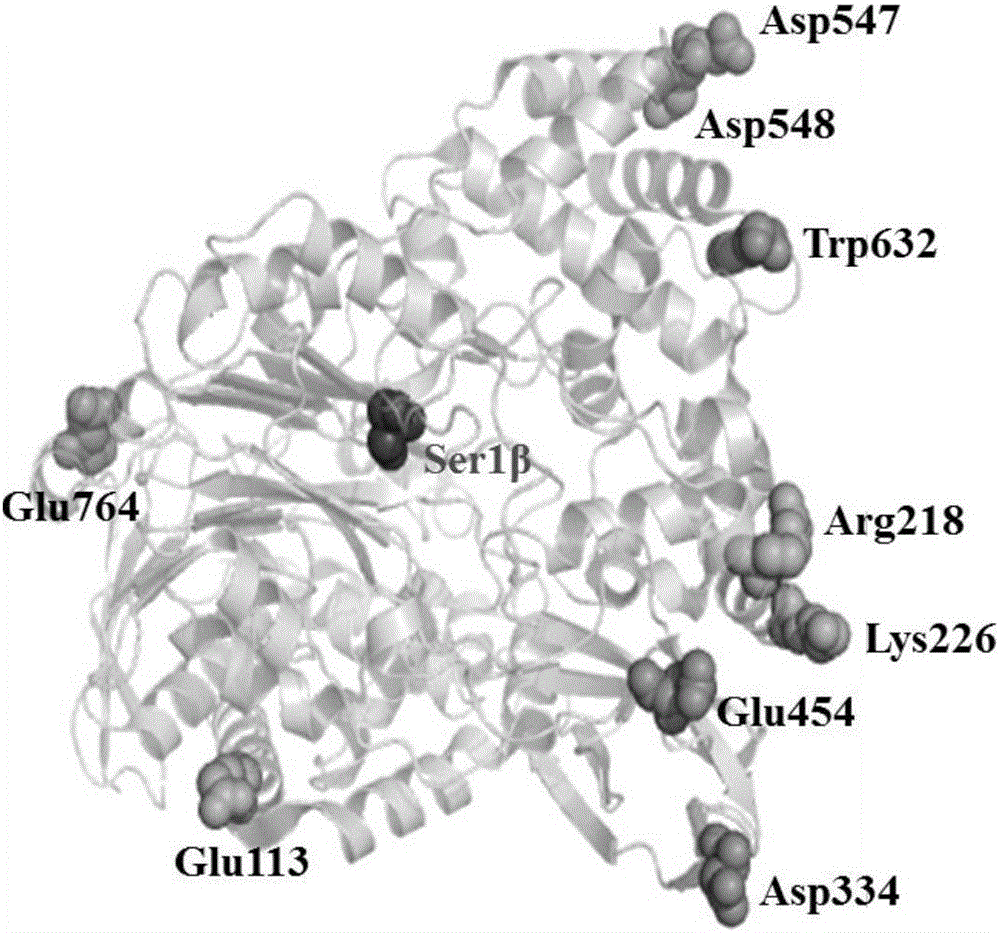
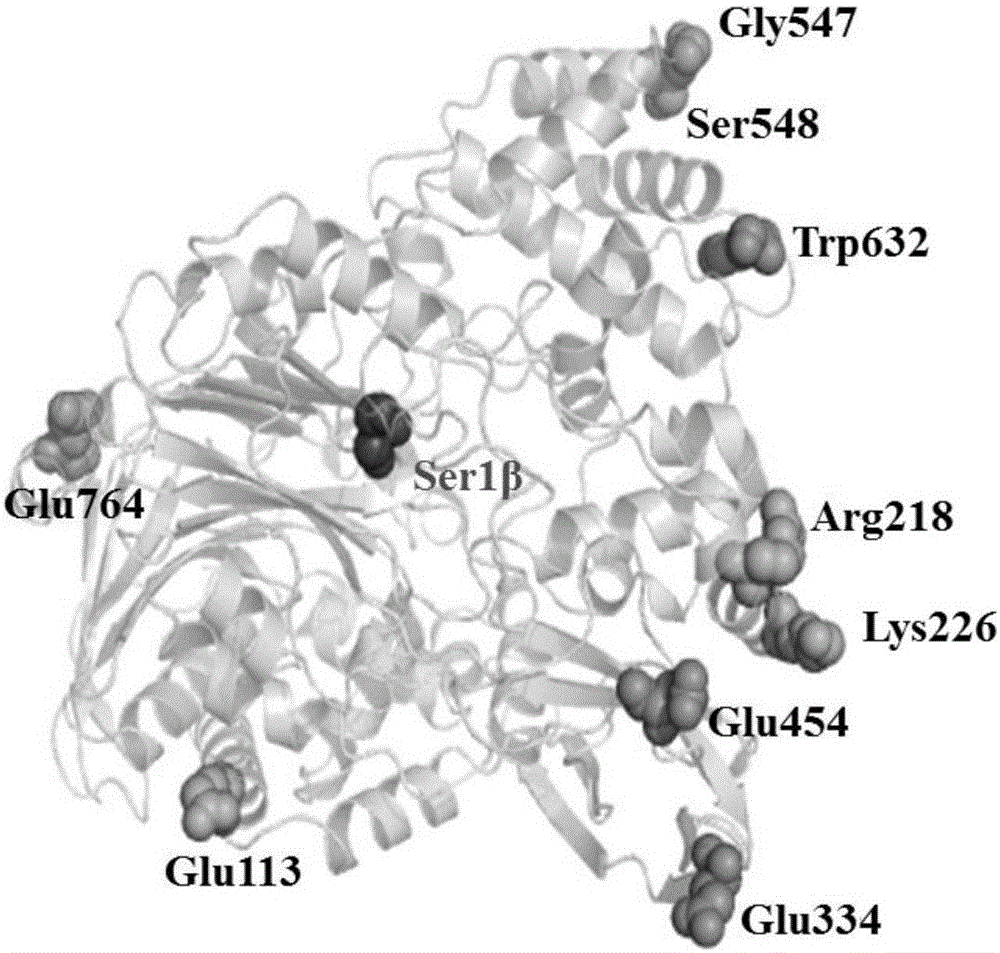
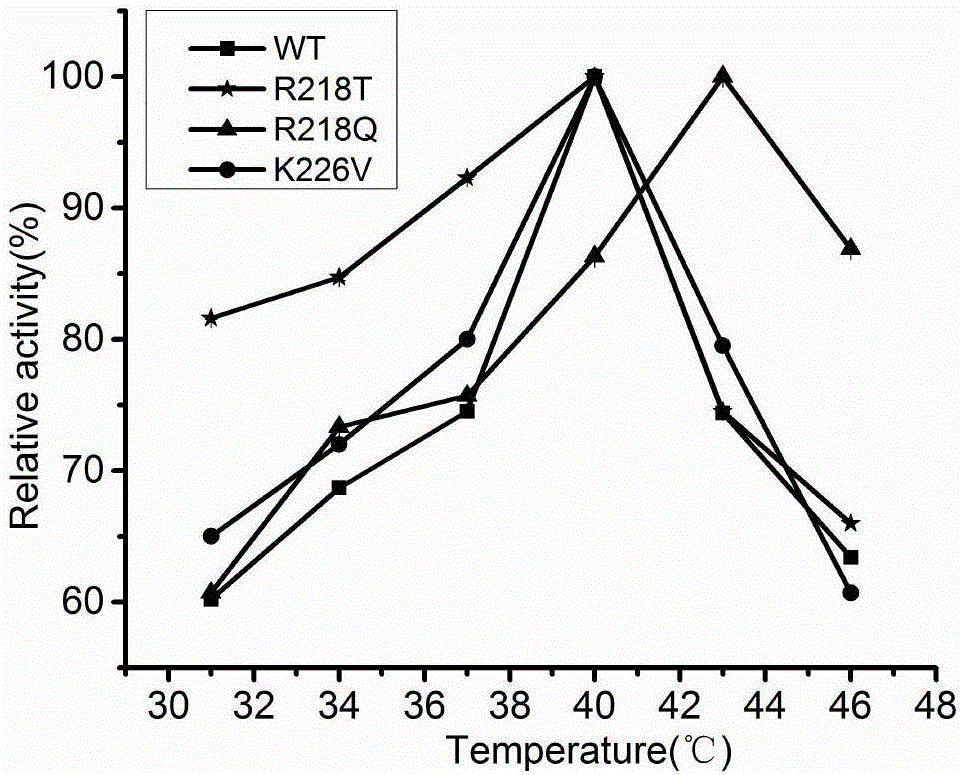
The invention discloses a cephalosporin C acylase mutant with higher heat stability. A mutation site of the cephalosporin C acylase mutant is selected from any one of No.113 locus glutamic acid, No.218 locus arginine, No.226 locus lysine, No. 334 locus glutamic acid, No.454 locus glutamic acid, No.547 locus glycine and No.632 locus tryptophan of an amino acid sequence shown as SEQ ID NO: 1. The invention also provides a construction method for the mutant. A mutation hotspot is selected on the basis of the analysis for a high B factor locus on the enzymatic structure. Target protein is selected after a genetic engineering method is adopted for introducing fixed locus saturated mutation. The invention has the advantages and beneficial effects that the mutant with the half-life period obviously longer than that of the wild cephalosporin C acylase is acquired, the industrial production can be more effectively adapted, the source for acquiring the cephalosporin C acylase mutant can be expanded according to a homological modeling method provided by the invention and the probability of selecting the mutant meeting the requirement can be increased.
Owner:WUHAN HANHAI NEW ENZYMES BIOLOGICAL TECH CO LTD
Extraction method of cephalosporin C
ActiveCN104278071AHigh light transmittanceReduce lossesOrganic chemistryFermentationUltrafiltrationTransmittance
An extraction method of cephalosporin C mainly comprises the steps of primary ultrafiltration, secondary ultrafiltration, macroporous resin adsorption, weakly alkaline resin adsorption and nanofiltration concentration. An ultrafiltration combination technology is adopted to directly filter a fermentation liquid, and the pH value of the obtained clarified filtrate is adjusted by sulfuric acid. The technology effectively reduces the loss of cephalosporin C in the acid adjustment process. The filtrate obtained through the ultrafiltration combination technology has the advantages of good light transmittance, and thorough removal of proteins and other impurities, and the method has the advantages of product quality improvement, increase of the extraction yield of a resin workshop section, and prolongation of the recovery cycle and the service life of resin, and obtained 7-ACA (7-aminocephalosporanic acid) has good quality and good stability.
Owner:SHANGHAI KAIXIN ISOLATION TECH CO LTD
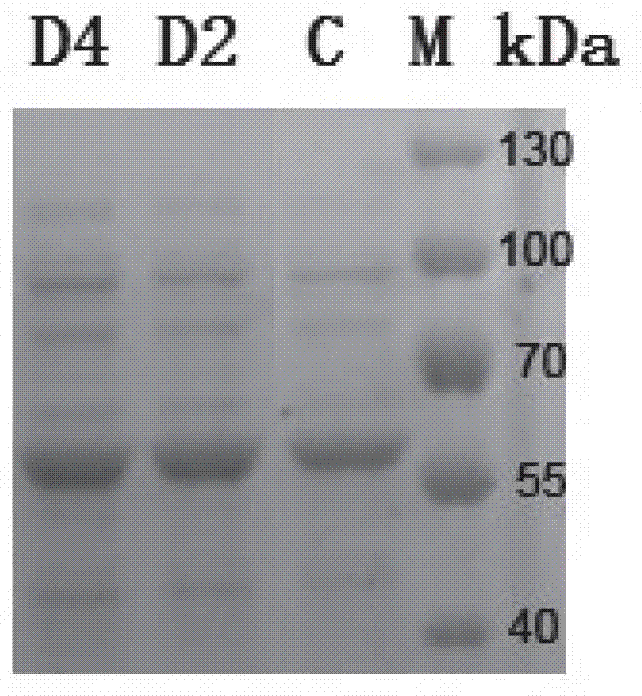
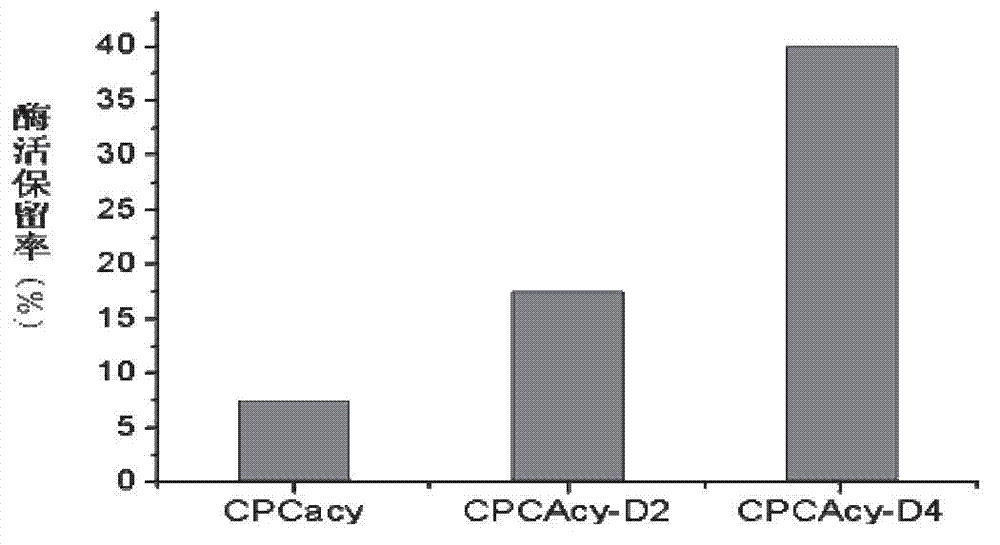
Mutated cephalosporin C acylase
The invention discloses a product-resistant inhibitory mutated cephalosporin C acylase, a gene carrier and a transformant of the enzyme, and application of the enzyme in one-step enzymatic production of 7-aminocephalosporanic acid, belonging to the technical field of enzyme engineering and biotechnology industry. The amino acid sequence of the enzyme is obtained by conducting deletion mutation on the amino acid sequence of cephalosporin C acylase coded by the gene sequence SEQ ID NO:1 to obtain enzyme CPCAcy-D2 and CPCAcy-D4. The amino acid sequences are shown as SEQ ID NO:2 and SEQ ID NO:3, respectively. The invention also discloses the gene carrier, the transformant, and the application of the enzyme. The enzyme has high expressing activity, and also the tolerance of the product is improved significantly, so that CPC is catalyzed efficiently to produce 7-ACA.
Owner:TSINGHUA UNIV
Method for detecting residual titer of cephalosporin C in cephalosporin residues
InactiveCN108267521AHigh sensitivityImprove separation efficiencyComponent separationSodium acetateSilica gel
The invention discloses a method for determining the residual titer of cephalosporin C in cephalosporin residues. The method comprises the following steps: (1) preparing a test solution: taking and grinding the cephalosporin residues, performing extraction by using a phosphate buffer solution, centrifuging the obtained solution, and taking the obtained supernatant to obtain the test solution; (2)preparing a reference solution: dissolving the cephalosporin C sodium salt in a mobile phase A to obtain the reference solution, wherein the mobile phase A is a sodium acetate buffer solution or an ammonium acetate buffer solution; and (3) detecting the residual tilter: taking the test solution, detecting the peak area by high performance liquid chromatography, and calculating the residual tilterof the cephalosporin C according to the peak areas of a reference substance and a sample, wherein the chromatographic conditions of the high performance liquid chromatography are as follows: the mobile phase is composed of the mobile phase A and a mobile phase B, the mobile phase B is acetonitrile, and a volume ratio of the mobile phase A to the mobile phase B is 97:3 to 99:1; and the chromatographic column is an octadecylsilane bonded silica gel column. The method has the advantages of high sensitivity, high separation efficiency, good selectivity and good repeatability.
Owner:YILI CHUANNING BIOTECH CO
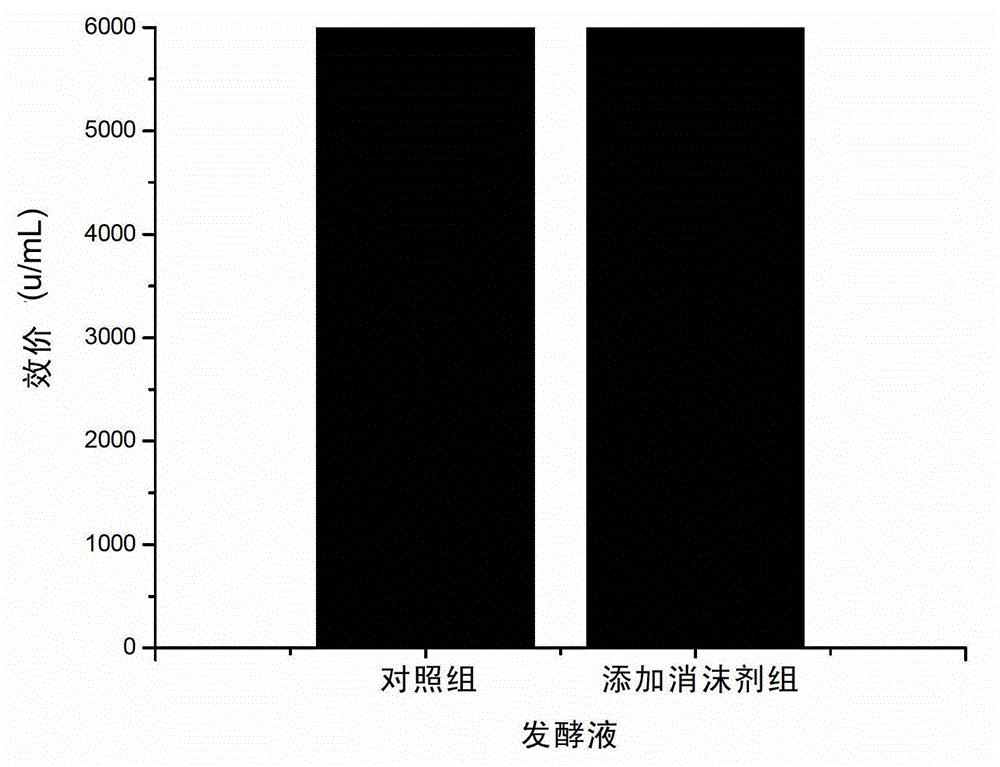
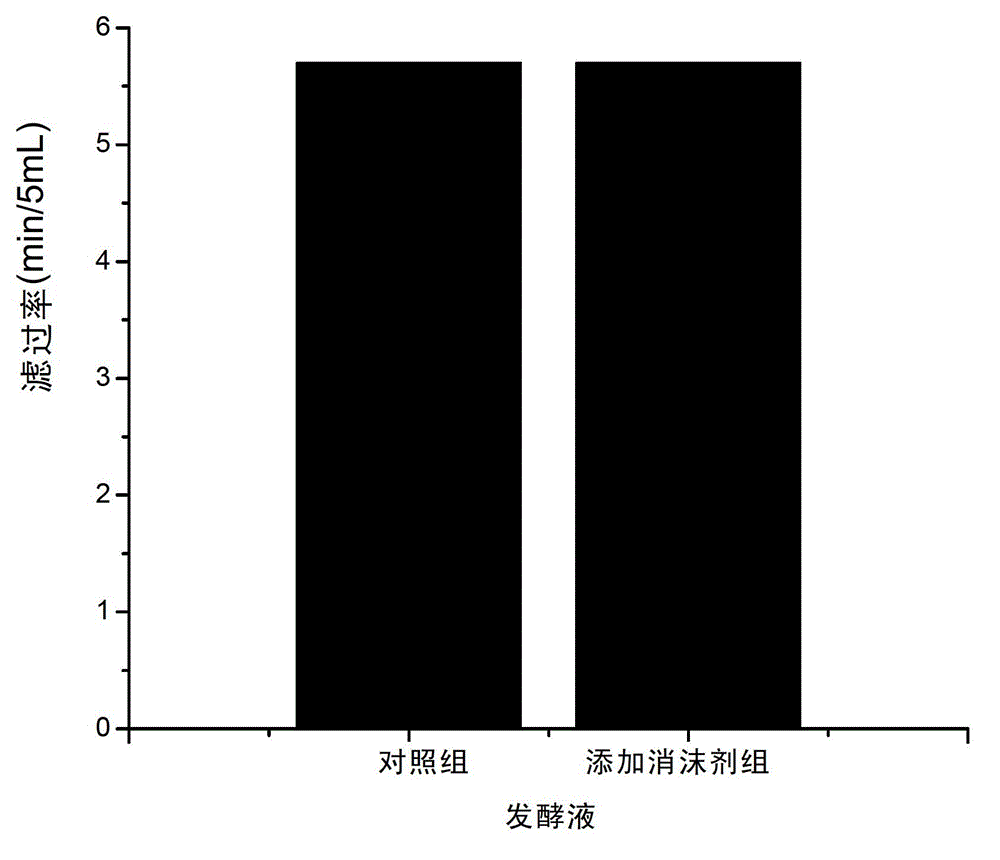
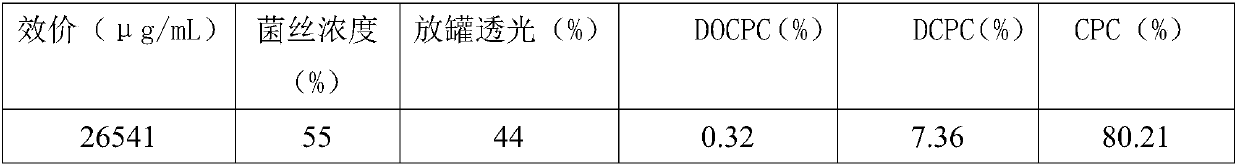
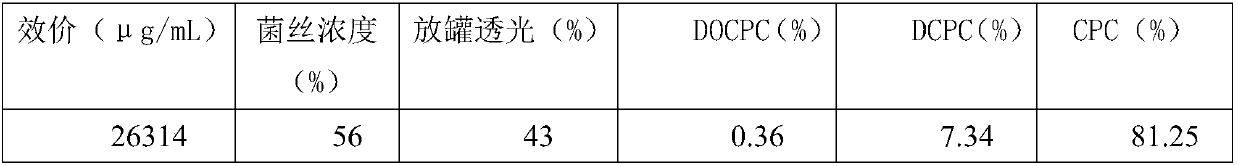
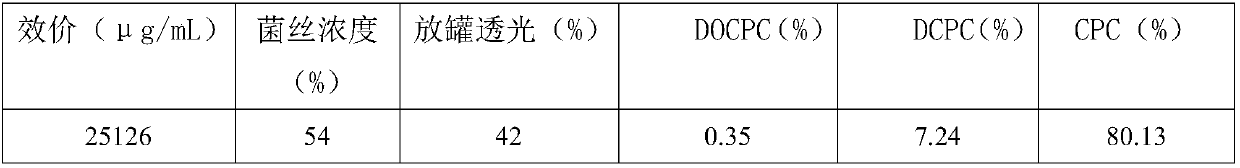
Fermentation process of cephalosporin C
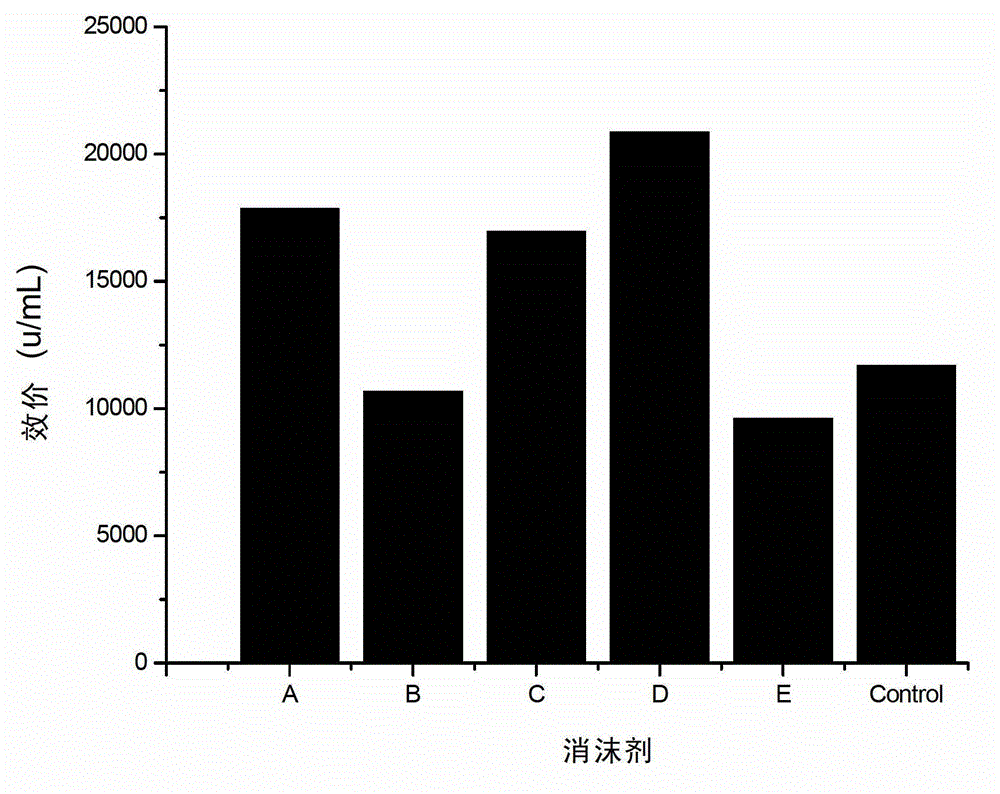
The invention relates to a fermentation process of cephalosporin C. At a later fermentation stage, the cephalosporin C is defoamed by supplementing a defoaming agent instead of soya-bean oil, so that cost of raw materials can be reduced by 15% under a condition that valence increasing and filtering velocity are not influenced.
Owner:SHANXI WEIQIDA PHARMA IND +1
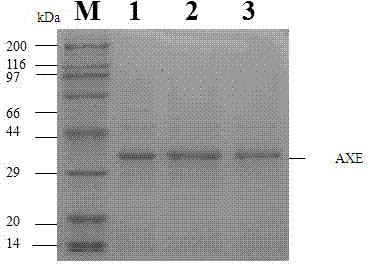
Acetylxylan esterase and application thereof
ActiveCN102952787AGood technical effectGreat application potentialHydrolasesFermentationAklanonic acidNucleotide
The invention discloses DNA (Deoxyribose Nucleic Acid) of a novel acetylxylan esterase, which has a nucleotide sequence shown as SEQ AXENO.1 and an amino acid sequence shown as SEQAXENO.2. Highest activity is obtained when a protein structure is a homotrimer. The invention also discloses a cloning vector containing the DNA of the novel acetylxylan esterase, a cloning technology and application of a recombinant esterase to hydrolysis of a short-chain fatty acid and acyl saccharides and application of the recombinant esterase to production of deacetylated 7-aminocephalosporanic acid and deacetylated cephalosporin C which are important intermediates of beta-lactam antibiotics; and the novel acetylxylan esterase has an important industrial application value.
Owner:NANJING UNIV OF TECH
Cephalosporin C mushroom dreg treatment method, hydrolysate and application thereof
ActiveCN108048519AIncrease productionReduce manufacturing costMicroorganism based processesFermentationHydrolysateMushroom
The invention provides a cephalosporin C mushroom dreg treatment method. The method includes following steps: a, taking cephalosporin C mushroom dreg, and adjusting water content to 20-60%; b, adding4-8mol / L of sulfuric acid solution, maintaining at 70-90 DEG C for 10-20h, and adjusting pH to 7.0-8.0. The invention further provides hydrolysate obtained through treatment. In addition, the invention further provides a cephalosporin C culture medium and a fermentation method. By the mushroom dreg treatment method, the problem of environment pollution caused by discharging of waste mushroom dregcan be solved, good nutritional substances are provided for cephalosporin C production, production cost of cephalosporin C can be lowered greatly, and industrial application prospect is good.
Owner:YILI CHUANNING BIOTECH CO
Removing deacetylase contaminant from cephalosporin C acylase using cationic surfactant
InactiveUS6630338B2High purityHigh productBioreactor/fermenter combinationsBiological substance pretreatmentsProteinase activityIon-exchange resin
An enzyme in a mixture containing the enzyme and a contaminant enzyme is purified by selectively aggregating and precipitating the contaminant enzyme with a surfactant. A deacetylase contaminant is separated from Cephalosporin C acylase using a cationic surfactant in an amount of 0.1 to 0.6%. Surfactants include benzylated or methylated cationic surfactants such as alkyl(palm)dimethylbenzyl ammonium chloride, alkyl(palm)trimethyl ammonium chloride and dodecyltrimethyl ammonium chloride. An immobilized enzyme is regenerated using a protease to remove the enzyme from a carrier. The carrier may be a synthetic adsorbent or an ion exchange resin having pores of 100 nm or less in diameter, and the immobilized enzyme is optionally crosslinked. Immobilized cephalosporin C acylase is regenerated using an alkaline or acidic protease.
Owner:HENKEL IP & HOLDING GMBH
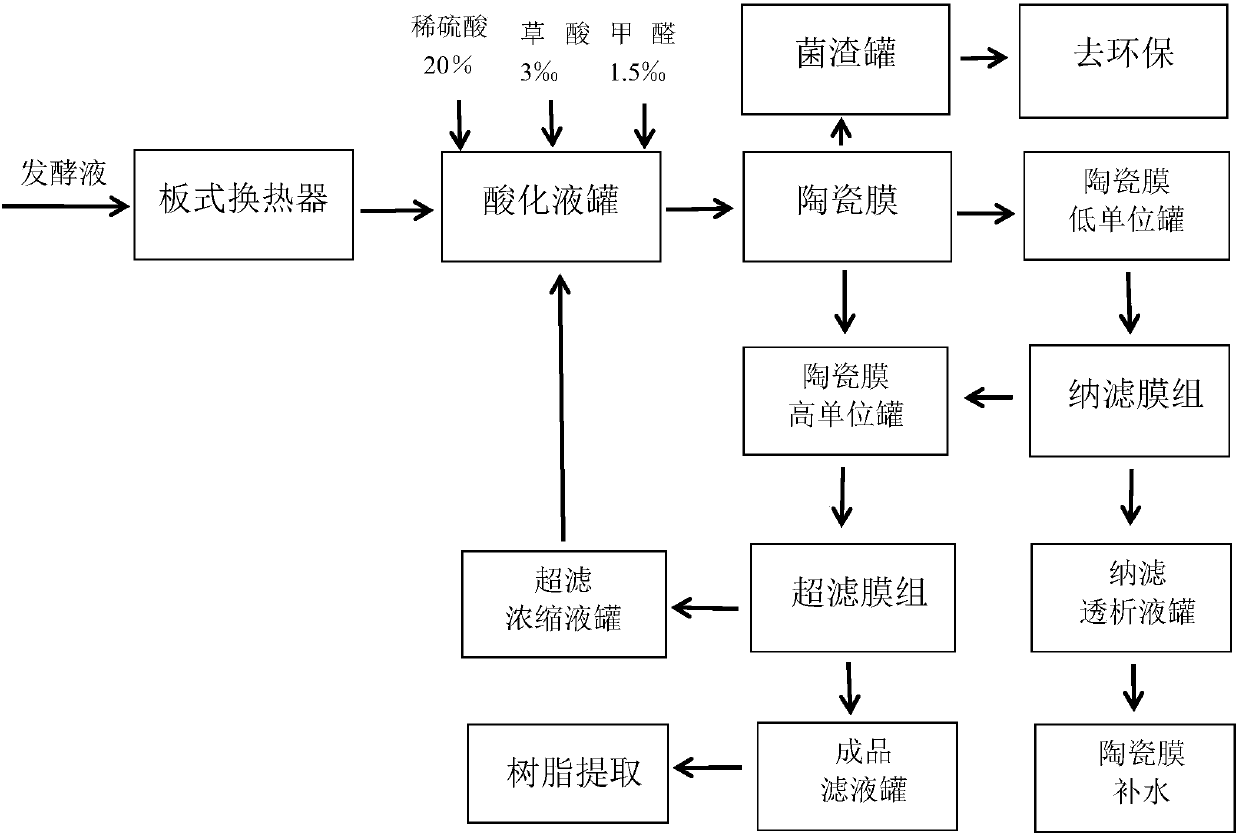
Method for purifying cephalosporin C fermentation broth
The invention discloses a method for purifying cephalosporin C fermentation broth. The method comprises the following steps: (1) taking the cephalosporin C fermentation broth, keeping the fermentationbroth at temperature of 5-25 DEG C, adjusting pH to be 2.2-3.2, adding 1-4permillagev / v formaldehyde and 1-5permillagew / w oxalic acid, and evenly mixing the mixture; (2) filtering the mixture by a ceramic membrane; (3) performing ultrafiltration; (4) performing nanofiltration to obtain a filtrate. The method is simple, efficient and easy to operate, problems existing in existing methods are effectively solved, and the method is suitable for popularization and application.
Owner:YILI CHUANNING BIOTECH CO
Method for improving anaerobic fermentation efficiency of antibiotic drug residues containing sporophore
ActiveCN105400704AHigh yieldReduce preprocessing timeFungiFermentationEcological environmentBiochemical engineering
The invention discloses a method for improving anaerobic fermentation efficiency of antibiotic drug residues containing sporophore. The method includes the step of anaerobic fermentation, and the step of culturing the antibiotic drug residues containing sporophore is further included before the step of anaerobic fermentation. Taking cephalosporin C drug residues for example, after the culture step is added, the yield of marsh gas is raised by 50% or more; the method can be further combined with ultrasound, microwave, thermokalite and the like for pretreatment, and the yield of marsh gas is also raised by 50% or more. By means of the method, the problems of recycling of a great number of antibiotic drug residues and environmental pollution can be solved, and the method has great significance in promoting sustainable development of the antibiotics pharmaceutical industry and protecting the ecological environment and has actual economic and social benefits and wide application prospect.
Owner:BIOLOGY INST OF HEBEI ACAD OF SCI
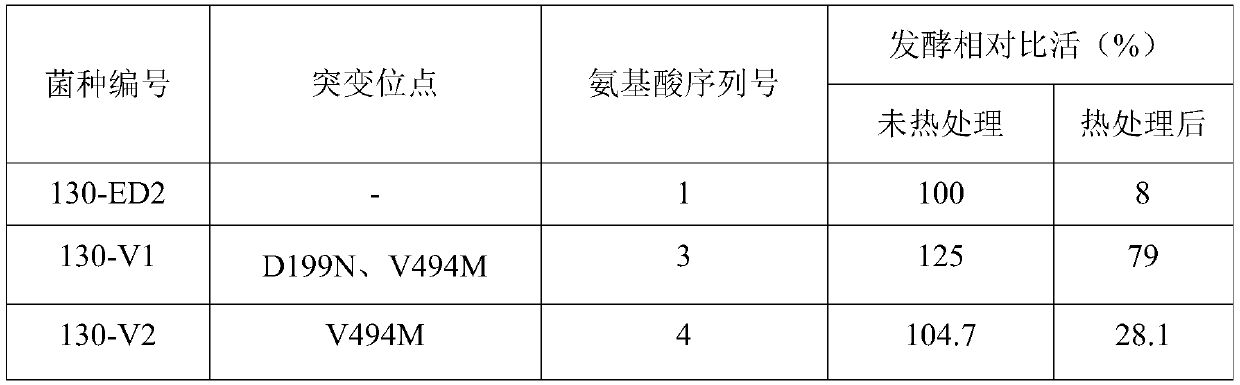
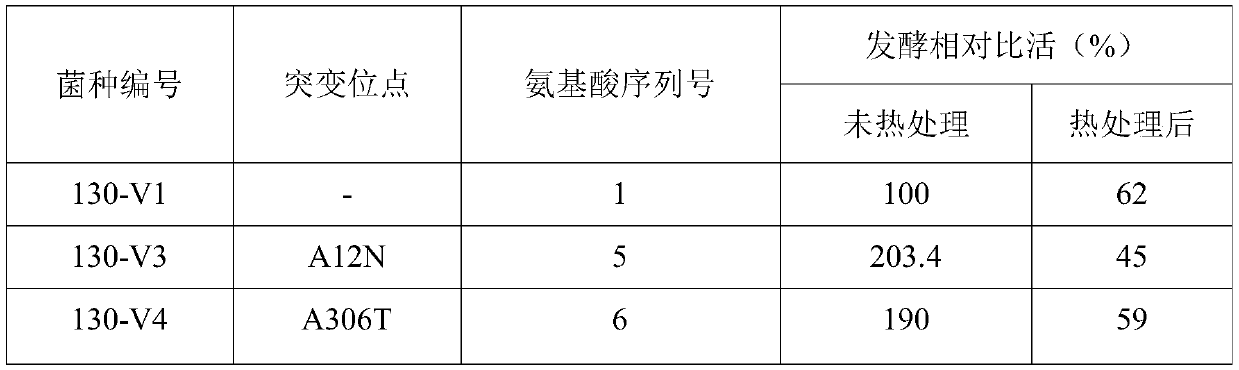
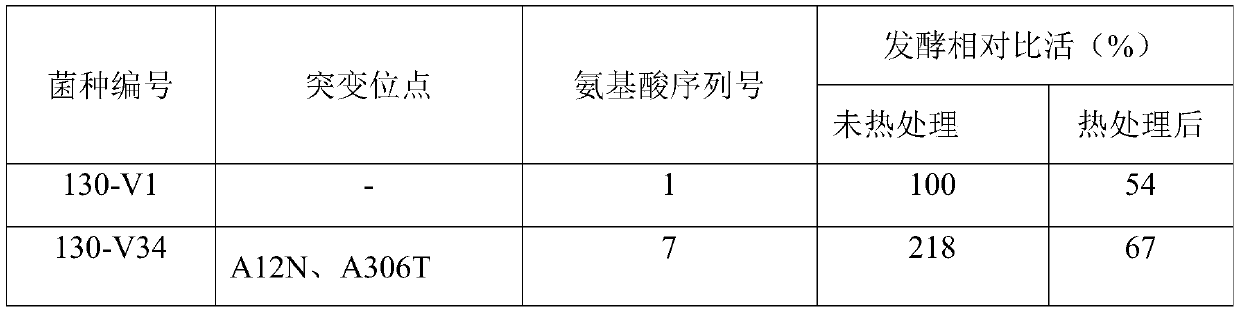
Cephalosporin C acylase mutant with high thermal stability
ActiveCN111172142AImprove thermal stabilityIncrease enzyme activityFungiBacteriaAklanonic acidPoint mutant
The invention discloses a cephalosporin C acylase mutant with a high thermal stability constructed by point mutation, which has an amino acid sequence selected from SEQ ID Nos: 3-7. Compared with initial cephalosporin C acylase with SEQ ID No: 1, the cephalosporin C acylase mutant disclosed by the invention has high thermal stability and higher enzyme activity, so that the cephalosporin C acylasemutant can be used for producing 7-aminocephalosporanic acid by adopting an one-step enzymatic method.
Owner:SHANGHAI TAOYUSHENG BIOTECHNOLOGY CO LTD
Immobilized cephalosporin C acylase and preparation method thereof
ActiveCN106148313ASolve the problem of high production costSuitable for industrial productionOn/in organic carrierBuffer solutionEnzyme
The invention discloses immobilized cephalosporin C acylase and a preparation method thereof. The immobilized cephalosporin C acylase comprises ES-1 resin and cephalosporin C acylase fixed on the ES-1 resin. The preparation method comprises steps as follows: the ES-1 resin and the cephalosporin C acylase are dissolved in a phosphate buffer solution with the concentration being 0.5-1.0 mol / L and the pH being 7.0-10.5 and immobilized at the temperature of 15-35 DEG C for 8-20 h. Compared with the prior art, the provided method for preparing the immobilized cephalosporin C acylase is short in immobilizing time and high in enzyme activity recovery rate, the problem of high production cost in the prior art is solved, and the method is more suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND +1
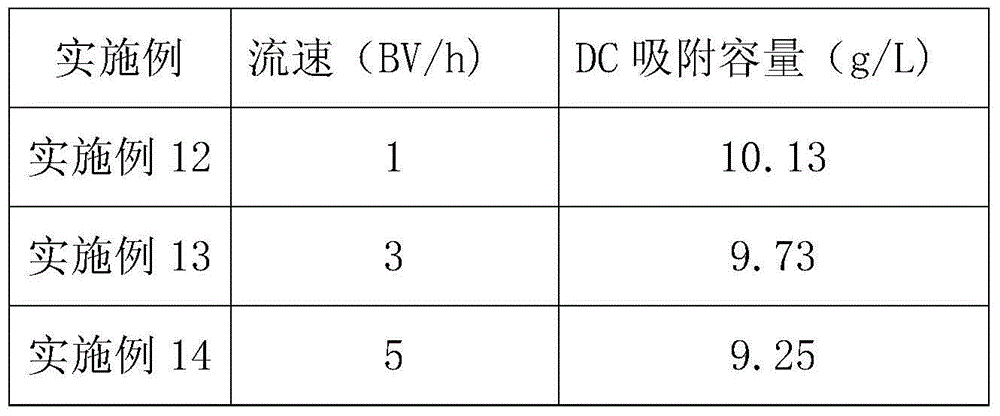
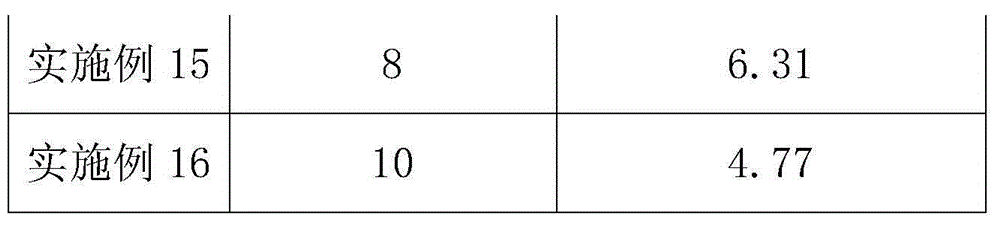
Method for recycling DCPC (deacetyl cephalosporin C) from cephalosporin C resin adsorption waste liquor
The invention provides a method for recycling DCPC from cephalosporin C resin adsorption waste liquor. According to the method, an anion exchange resin 1 is firstly used for removing residual inorganic anions, particularly sulfate ions, in the adsorption raffinate; and the remaining feed liquid is adsorbed by a macroporous adsorption resin, a salt solution is used as desorption agent, the elutriant is directly concentrated by nanofiltration after being decolored through an anion exchange resin 2, further, after the processes of splitting decomposition, crystallization, drying, and so on, a D-7ACA qualified product with purity of not less than 98% and color grade of more than 4 is obtained. Resin extraction yield is not less than 85%, and total yield of the process is not less than 45%.
Owner:SUNRESIN NEW METERIALS CO LTD XIAN
Cephalosporin C acylase mutant with one or any point mutations and preparation method thereof
The invention relates to the field of production of medicine, in particular to production of cephalosporin antibiotics and a production enzyme therefor, more in particular to a cephalosporin C acylasemutant with one or any point mutations, nucleic acid to encode the mutant, a related expression vector and host cells, a preparation method of the mutant, and application of the mutant to prepare 7-ACA (7-aminocephalosporanic acid) through one-step lysis of CPC (cephalosporin).
Owner:CSPC SHENGXUE GLUCOSE CO LTD
Use of recommbined D-amino acid oxidase
A recombinative D- amino acid oxidase and its DNA code sequence. Activity to catalysis rhzomorphC of the enzyme is 25% higher than that of its parents at least.
Owner:BIORIGHT WORLDWIDE
Production process of dry cephalosporin C sodium salt
The invention discloses a production process of a dry cephalosporin C sodium salt. The production process comprises the following steps of: 1, performing nano-filtration concentration; 2, drying: conveying a concentrated solution into a centrifugal spray drier by using a screw pump, controlling an air inlet temperature at 155-165 DEG C, controlling the air outlet temperature at 75-90 DEG C, retaining a material in the tower chamber of a drying tower for 8-12 seconds, quickly extracting the material, separating and collecting with a cyclone separator, inspecting, and feeding the qualified material to packaging equipment, wherein inspection qualification standards are that: (1) property: slightly yellow crystalline powder; (2) moisture is less than or equal to 6.0 percent; (3) a color grade is less than or equal to Y-7; and (4) the purity of cephalosporin C is more than or equal to 86 percent; and III, packaging: metering a finished product, bagging, sealing and warehousing. The production process has the beneficial effects that: (1) the process flow is short, and the production efficiency is high; and (2) a large amount of alcohol is saved, production cost is reduced, and potential safety hazard is reduced.
Owner:SHANDONG LIHAIRUN BIOLOGICAL TECH
Method for fermentation production of cephalosporin C
ActiveCN106191192AImprove single batch outputLow costMicroorganism based processesFermentationFermentation brothAcremonium
The invention provides a method for fermentation production of cephalosporin C. The method comprises a step a of taking cephalosporins acremonium, and preparing primary seeds and secondary seeds; a step b of taking the secondary seeds prepared through the step a, inoculating the secondary seeds to a cephalosporins acremonium fermentation medium to perform fermentation, performing discharging after fermentation for 50-115 h, and re-cultivating the discharged liquid; a step c of taking the fermentation broth and the discharged culture liquid obtained through the step b, performing separation and purification, and obtaining cephalosporin C. According to the fermentation method, the single batch yield of cephalosporin C can be remarkably improved, the cost is greatly reduced, and the industrial application prospect is good.
Owner:上海锐康生物技术研发有限公司
Method for producing functional organic fertilizer by using cephalosporin C filter residues
InactiveCN109776238AAll environmentally friendly treatmentThorough environmental protectionFertilizer mixturesMasterbatchTrace element
The invention relates to the technical field of fertilizer production, and particularly discloses a method for producing a functional organic fertilizer by using cephalosporin C filter residues. Withthe cephalosporin C filter residues as a raw material, the method for producing the functional organic fertilizer by using the cephalosporin C filter residues is characterized by comprising the stepsof adding strong acid to the raw material for hydrolysis to obtain a hydrolyzate; adding alkali to the hydrolyzate for neutralization, and conducting heat preservation to obtain an organic fertilizermasterbatch; conducting vacuum concentration on the organic fertilizer masterbatch, adding medium and trace elements, and through fine filtration, obtaining a filtrate, namely the functional organic fertilizer product. By means of the method, the treatment problem of the cephalosporin C filter residues is thoroughly solved, the process is simple, the cost is low, the product functionality is high,the market prospect is good, and the method has good social and economic benefits and has industrial popularization prospects.
Owner:HEZE RUIZHI TECH DEV
Cephalosporin C acylase mutant as well as preparation method and application thereof
ActiveCN112662655A1 Increased enzyme activityHigh enzyme activity efficiencyHydrolasesFermentationArginineAklanonic acid
The invention belongs to the technical field of bioengineering, and particularly relates to a cephalosporin C acylase mutant as well as a preparation method and application thereof. The cephalosporin C acylase mutant has an amino acid sequence as shown in SEQ ID NO: 2, wherein the amino acid sequence shown as SEQ ID NO: 2 is obtained by carrying out substitution mutation on the 245-247th amino acid of the amino acid sequence shown as SEQ ID NO: 1; the tyrosine at the 245th site is mutated into phenylalanine, the arginine at the 246th site is mutated into methionine, and the leucine at the 247th site is mutated into serine. The enzyme activity of the cephalosporin C acylase mutant disclosed by the invention is improved by 210 times or more, compared with that of wild type GL-7-ACA acylase SED ID NO: 1, and can be applied to one-step enzymatic production of 7-aminocephalosporanic acid (7-ACA).
Owner:SHANDONG JINCHENG KERUI CHEMICAL CO LTD
Method for preparing cephalosporin C acylase immobilized enzyme
ActiveCN104593351AFlocculation achievedGood removal effectAntibacterial agentsHydrolasesEscherichia coliPhosphate
The invention provides a method for preparing a cephalosporin C acylase immobilized enzyme. The method comprises the following steps: (1) dialyzing with phosphate buffer liquor after concentrating escherichia coli fermentation liquor through a ceramic membrane, and performing high-pressure crushing to obtain cell crushing liquor; (2) adding a mixed flocculating agent consisting of aluminum sulfate, ferrous sulfate and neutral polyacrylamide into the cell crushing liquor, and flocculating at a room temperature; adding diatomite and centrifuging to collect liquid supernatant; (3) heating after adding composite sulfate into the liquid supernatant; after cooling to the room temperature, adding the diatomite and centrifuging to collect liquid supernatant; (4) concentrating through a hollow fiber membrane made of a polyacrylonitrile material and dialyzing with phosphate buffer liquor to obtain concentrated enzyme liquor; and (5) adding an immobilization carrier into the concentrated enzyme liquor, and performing suction filtration after sufficiently combining the enzyme with the carrier to obtain the cephalosporin C acylase immobilized enzyme. The invention further discloses a cephalosporin C acylase immobilized enzyme prepared by the method and an application thereof.
Owner:ANHUI BBCA FERMENTATION TECH ENG RES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com