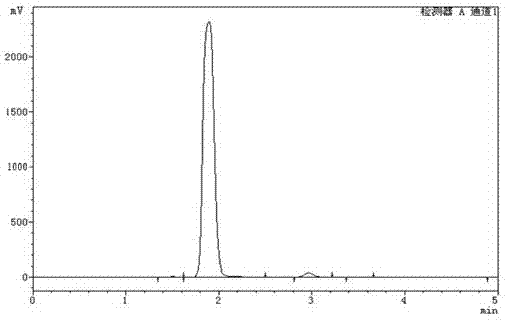
Technology for preparing medicine intermediate D-7-ACA by two enzyme carriers one-step method
A technology of D-7-ACA and preparation process, which is applied in the technical field of preparing D-7-ACA by two-enzyme one-step method, can solve the problems of low production safety, long production cycle, poor product quality and the like, and shorten production The effect of cycle time, less by-products and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The process for preparing pharmaceutical intermediate D-7-ACA by two-enzyme one-step method, the specific steps include:
[0045] (1) According to the potency of cephalosporin C sodium salt concentrate determined by high performance liquid chromatography, dilute the cephalosporin C sodium salt concentrate to 28000u / mL, and adjust the pH value to 6.5 with 5% ammonia water Finally, add 500mL of the above dilution into an enzyme cleavage tank containing 40.23g of immobilized CPC acylase (enzyme activity 87u / g) and 8.75g immobilized deacetylesterase (enzyme activity 400u / g) , at 20°C, the pH value of the lysate was adjusted to 8.2 by using ammonia water with a mass fraction of 5.5% through an enzyme cleavage tank, and the final lysate was obtained after the reaction was completed;
[0046] (2) Transfer the final lysate obtained in step (1) to a crystallization tank, cool down to 3°C, add dropwise a 12% hydrochloric acid solution to adjust the pH to 3.5, grow crystals at 0°C...
Embodiment 2
[0048] The process for preparing pharmaceutical intermediate D-7-ACA by two-enzyme one-step method, the specific steps include:
[0049] (1) According to the titer of cephalosporin C sodium salt concentrate determined by high performance liquid chromatography, dilute the cephalosporin C sodium salt concentrate to 30000u / mL, and adjust the pH value to 7.0 with 5% ammonia water Finally, add 500mL of the above dilution into an enzyme cleavage tank containing 41.05g of immobilized CPC acylase (enzyme activity: 87 u / g) and 8.93g immobilized deacetylase (enzyme activity: 400 u / g) At 18°C, the pH of the lysate was adjusted to 8.0 by using ammonia water with a mass fraction of 8% through an enzyme cleavage tank, and the reaction was completed to obtain the final lysate;
[0050] (2) Transfer the final lysate obtained in step (1) to a crystallization tank, cool down to 0°C, add dropwise a 20% hydrochloric acid solution to adjust the pH to 3.0, grow crystals at 5°C for 1.5 hours, filter...
Embodiment 3
[0052] The process for preparing pharmaceutical intermediate D-7-ACA by two-enzyme one-step method, the specific steps include:
[0053] (1) According to the potency of cephalosporin C sodium salt concentrate determined by high performance liquid chromatography, dilute the cephalosporin C sodium salt concentrate to 32000u / mL, and adjust the pH value to 7.5 with 5% ammonia water Finally, add 500mL of the above dilution into an enzyme cleavage tank containing 40.87g of immobilized CPC acylase (enzyme activity: 87 u / g) and 8.89g immobilized deacetylase (enzyme activity: 400 u / g) In 25 ° C, the pH of the lysate was adjusted to 8.5 by using ammonia water with a mass fraction of 4.5% through the enzyme cleavage tank, and the final lysate was obtained after the reaction was completed;
[0054] (2) Transfer the final lysate obtained in step (1) to a crystallization tank, cool down to 10°C, add dropwise hydrochloric acid solution with a mass fraction of 10% to adjust the pH to 5.0, gro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com