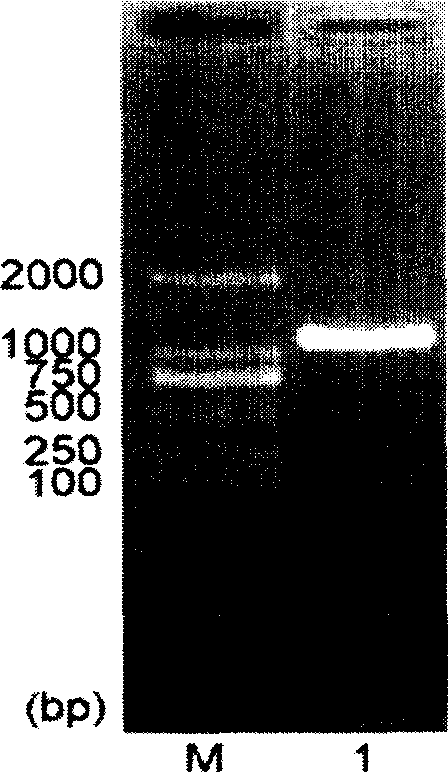Patents
Literature
83 results about "Point mutant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mutation penicillin G acylase, recombinant expression plasmid and transformation engineering strains thereof
ActiveCN101177688AImprove synthesis abilityMaximum conversion rate increaseBacteriaHydrolasesHydrolysatePolymerase L
The invention relates to a gene, mutant plasmid and engineering bacteria which have improved synthesis performance to penicillin G acylase and are obtained by a gene site-directed mutagenesis method, and mutant enzyme can also be obtained with improved synthesis performance to penicillin G acylase by fermenting and purifying the engineering bacteria. Two enzymes Kpn I and Pst I are firstly used for cutting pUC18 by the invention, then T4 polymerase is adopted to make the ends blunt, and pZ01 is obtained through self-linkage; the enzyme of EcoR I is used for cutting pZ01, and then connected with pEES102 that is also cut by the enzyme of EcoR I, thereby obtaining the recombinant plasmid pY020; the pY020 is adopted as a template plasmid, and TaKaRa MuTanBEST Kit is utilized for conducting the site-directed mutagenesis to B.megaterium PGA, thereby obtaining the mutant plasmid with improved synthesis performance to the penicillin G acylase. The mutant plasmid is transformed to bacillus subtilis to obtain the required engineering bacteria. The engineering bacteria are amplified and fermented, and the mutant enzyme with improved maximum conversion rate of 7-ADCA and the ratio of synthetic product / hydrolysate can be obtained after the engineering bacteria are purified.
Owner:SHANXI WEIQIDA PHARMA IND
System for efficient multi-point interposition of unnatural amino acid into mammalian cells
ActiveCN107022568AEfficient modification purposeSsRNA viruses positive-senseVirus peptidesMammalian cellPoint mutant
The invention provides a system for efficient multi-point interposition of unnatural amino acid into mammalian cells. The system comprises an expression vector for expressing protein containing unnatural amino acid, wherein the unnatural amino acid is encoded by a codon UAG and contains a vector of methanogen tRNA (tRNAPyl) and pyrrolysyl-tRNA synthetase (PylRS); and the system also comprises an eRF1 cell line obtained from mutation. In addition, the invention also provides a method for preparing fixed-point mutant protein with the application of the system.
Owner:PEKING UNIV
Recombinant PAI-1 inhibitor, composition containing recombinant PAI-1 inhibitor, and uses of recombinant PAI-1 inhibitor and composition in treatment and detection
ActiveCN104789544AHas inhibitory effectAvoid interactionHydrolasesPeptide/protein ingredientsDepressantHydrolase
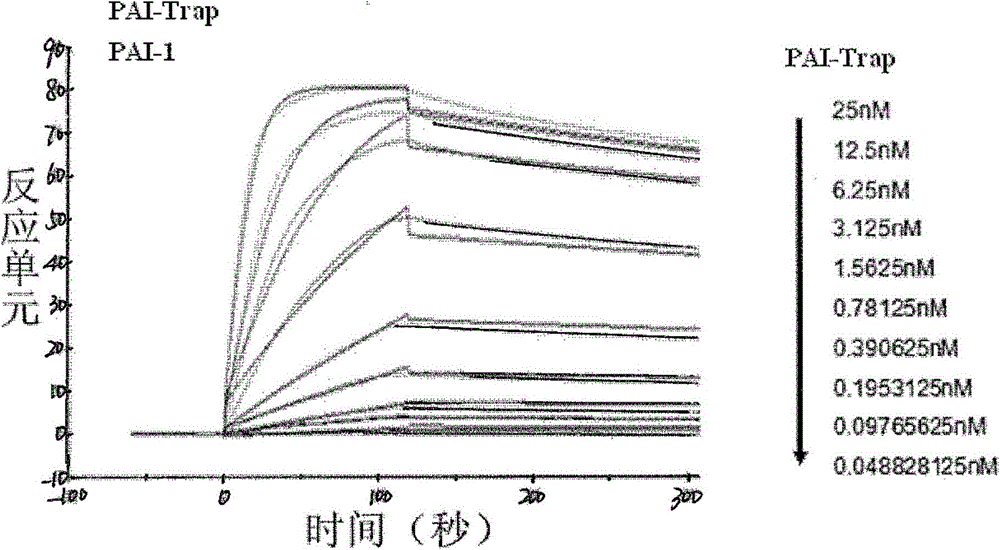
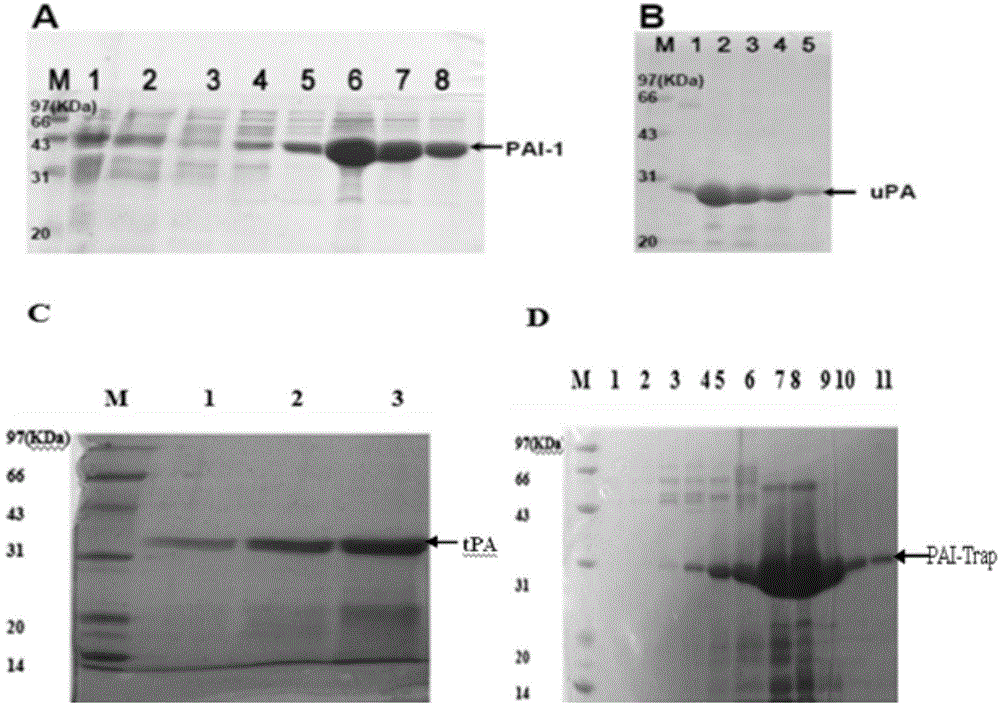
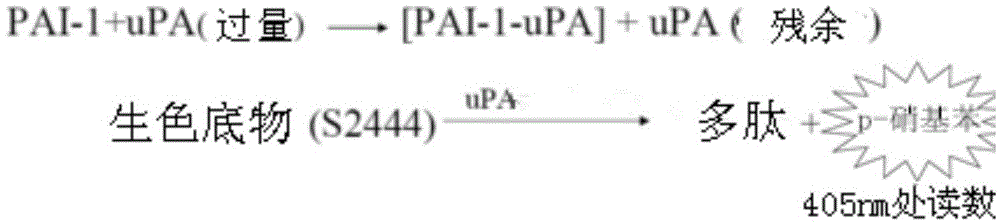
The present invention provides a recombinant PAI-1 inhibitor, which is an uPA hydrolase domain with S195A mutation or a PAI-1 hydrolase domain with S195A mutation, and has PAI-1 binding force. The present invention further provides a composition containing the inhibitor, and uses of the composition in preparation of treatment drugs and PAI-1 detection reagents. According to the present invention, the uPA or tPA hydrolase domain is modified to optimize the binding force with the PAI-1 so as to construct the recombinant PAI-1 inhibitor, wherein the recombinant PAI-1 inhibitor contains the least S195A point mutant.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
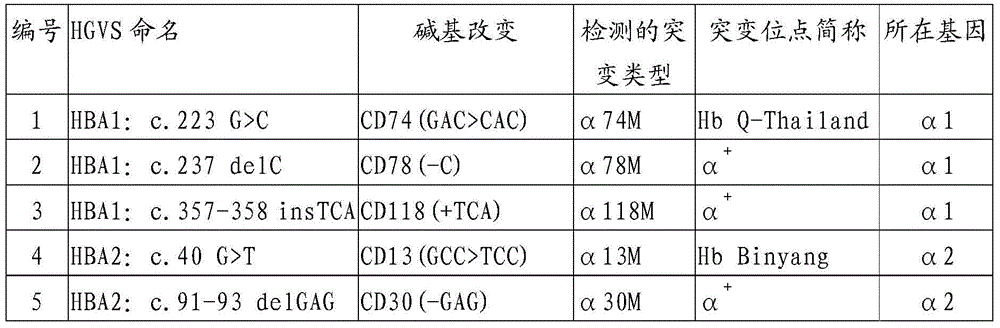
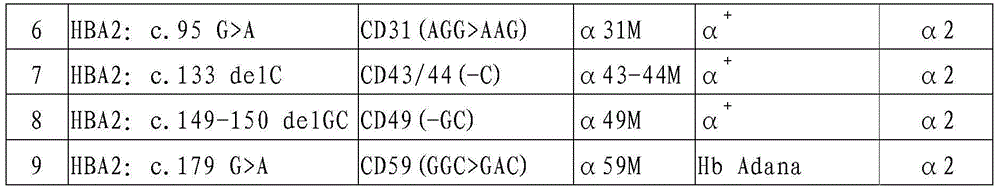
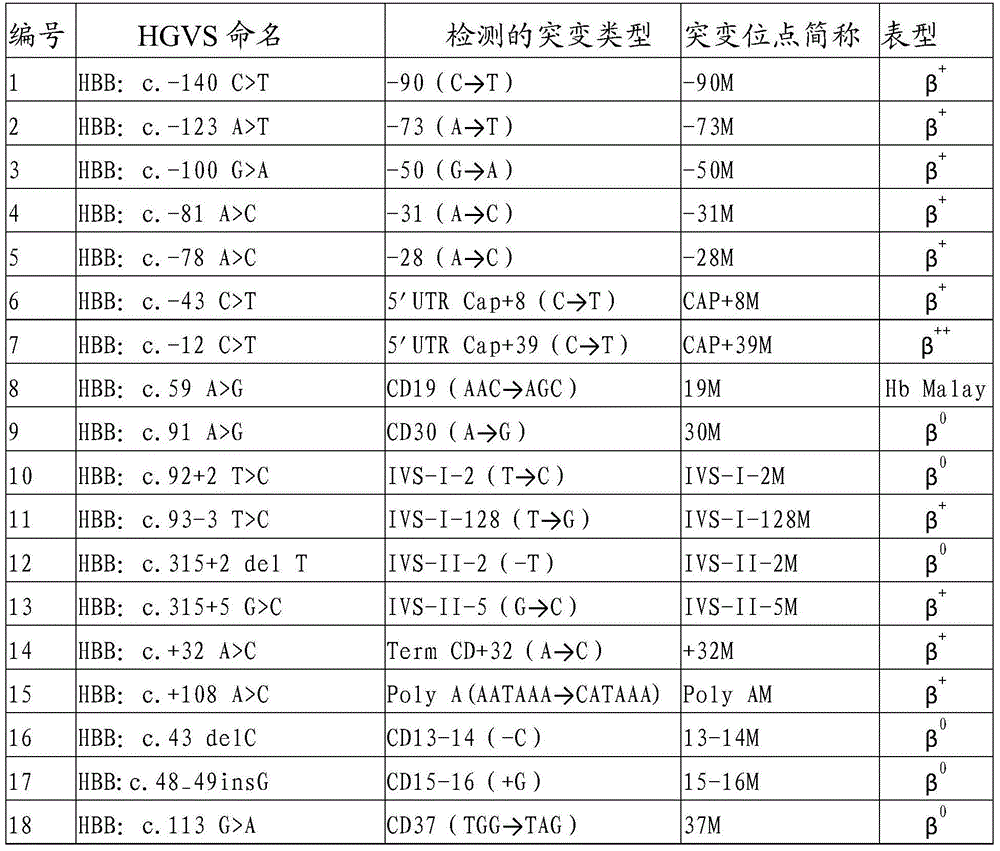
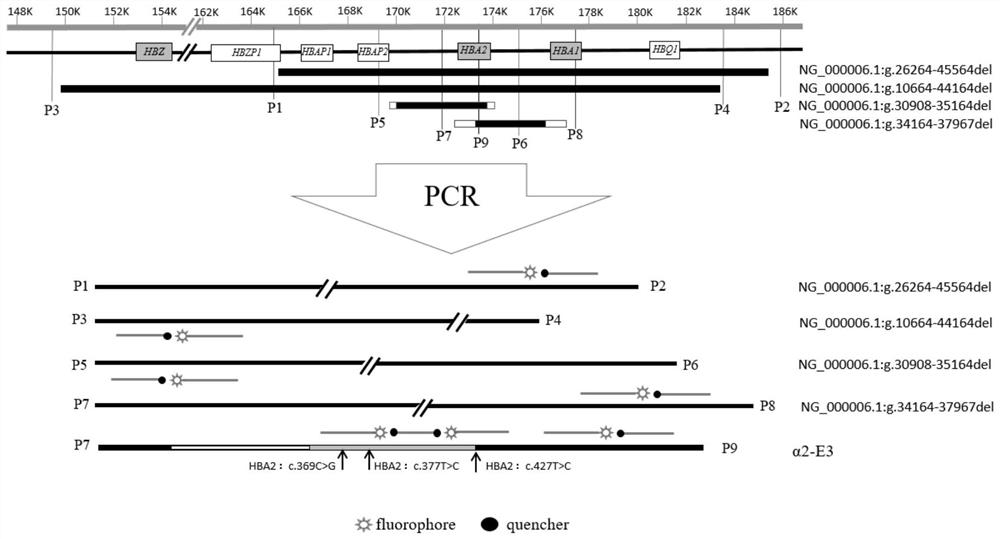
Gene chip and kit for detecting rare Mediterranean anemia genes
ActiveCN103602751AReduce missed detection rateReduce or avoid birthsMicrobiological testing/measurementNucleic Acid ProbesNormal control
The invention relates to a gene chip and kit for detecting rare Mediterranean anemia genes. The gene chip for detecting rare Mediterranean anemia genes comprises a substrate and oligonucleotide probes fixed on the substrate, wherein the oligonucleotide probes comprise normal control oligonucleotide probes disclosed as SEQ ID NO:1-2 corresponding to non-deletion alpha-Mediterranean anemia gene, normal control oligonucleotide probes disclosed as SEQ ID NO:3-4 corresponding to point mutant beta-Mediterranean anemia gene, oligonucleotide probes disclosed as SEQ ID NO:5-13 for detecting non-deletion alpha-Mediterranean anemia gene, and oligonucleotide probes disclosed as SEQ ID NO:14-40 for detecting point mutant beta-Mediterranean anemia gene. The gene chip and kit can detect 9 rare non-deletion alpha-Mediterranean anemia genes and 27 rare point mutant beta-Mediterranean anemia genes at one time, and create conditions for more comprehensive screening of point mutant Mediterranean anemia genes.
Owner:亚能生物技术(深圳)有限公司
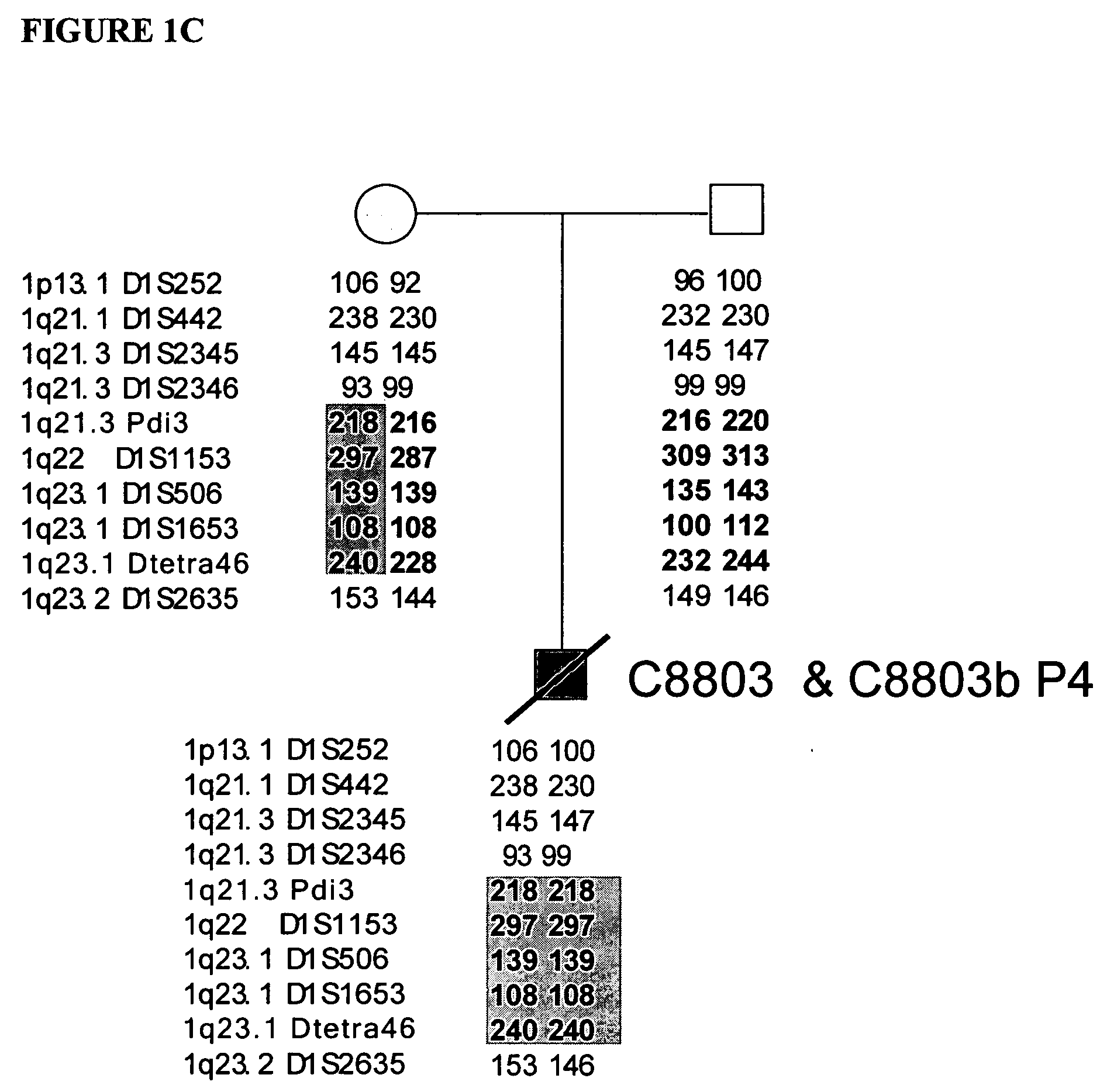
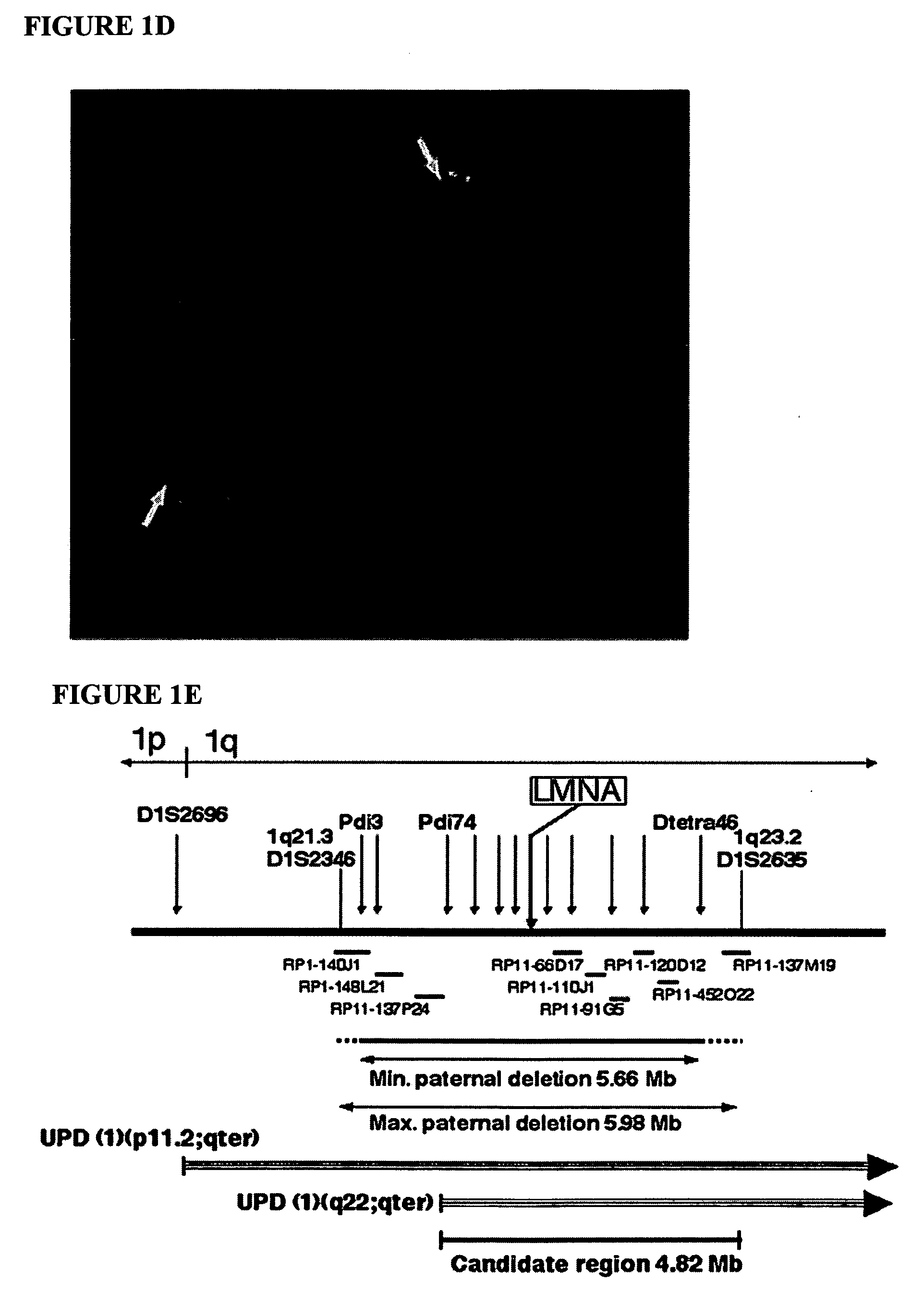
LMNA gene and its involvement in hutchinson-gilford progeria syndrome (HGPS) and arteriosclerosis
Disclosed herein are point mutations in the LMNA gene that cause HGPS. These mutations activate a cryptic splice site within the LMNA gene, which leads to deletion of part of exon 11 and generation of a mutant Lamin A protein product that is 50 amino acids shorter than the normal protein. In addition to the novel Lamin A variant protein and nucleic acids encoding this variant, methods of using these molecules in detecting biological conditions associated with a LMNA mutation in a subject (e.g., HGPS, arteriosclerosis, and other age-related diseases), methods of treating such conditions, methods of selecting treatments, methods of screening for compounds that influence Lamin A activity, and methods of influencing the expression of LMNA or LMNA variants are also described. Oligonucleotides and other compounds for use in examples of the described methods are also provided, as are protein-specific binding agents, such as antibodies, that bind specifically to at least one epitope of a Lamin A variant protein preferentially compared to wildtype Lamin A, and methods of using such antibodies in diagnosis, treatment, and screening. Also provided are kits for carrying out the methods described herein.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +2
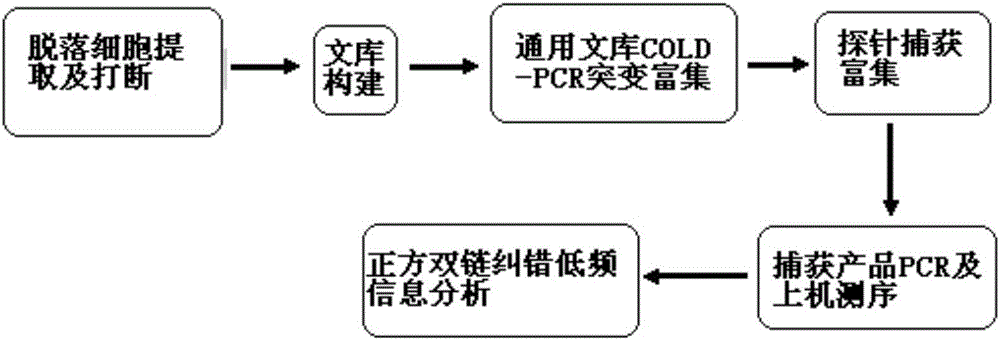
Method for low-frequency mutant-enriched sequencing of DNA of exfoliative cells
ActiveCN105132407AAccurate predictionLow costMicrobiological testing/measurementDNA preparationHuman DNA sequencingInformation analysis
The invention provides a method for low-frequency mutant-enriched sequencing of DNA of exfoliative cells. The method includes the steps of extraction of the DNA of the exfoliative cells, break of the DNA, sample DNA library construction, universal library TT-COLD PCR amplification enrichment, probe enrichment capture, captured product PCR and computer sequencing and positive-negative double-strand error-correction low-frequency information analysis, to be more specific, performing universal adapter primer based TT COLD PCR on all types of mutant to realize first-stage mutant-enriched amplification; designing an enrichment probe chip to replace a probe designed for a human genome reference sequence hg19 with a probe designed based on mutation bases for hot point mutants, keeping probes at other sites unchanged, and performing second-stage enriched capture. 12bp own sequences inserted into two ends of the DNA in library construction are taken as labels for positive and negative double-strand error correction comparison, data utilization rate is increased, low-frequency accurate detection is realized, and high-specificity detection for 0.01% low-frequency mutations can be achieved.
Owner:BEIJING GENEPLUS TECH
Improved overlap extension PCR process and mutation gene obtained thereby
InactiveCN1861799AImprove fidelityPromote accumulationFermentationPcr methodSite-directed mutagenesis
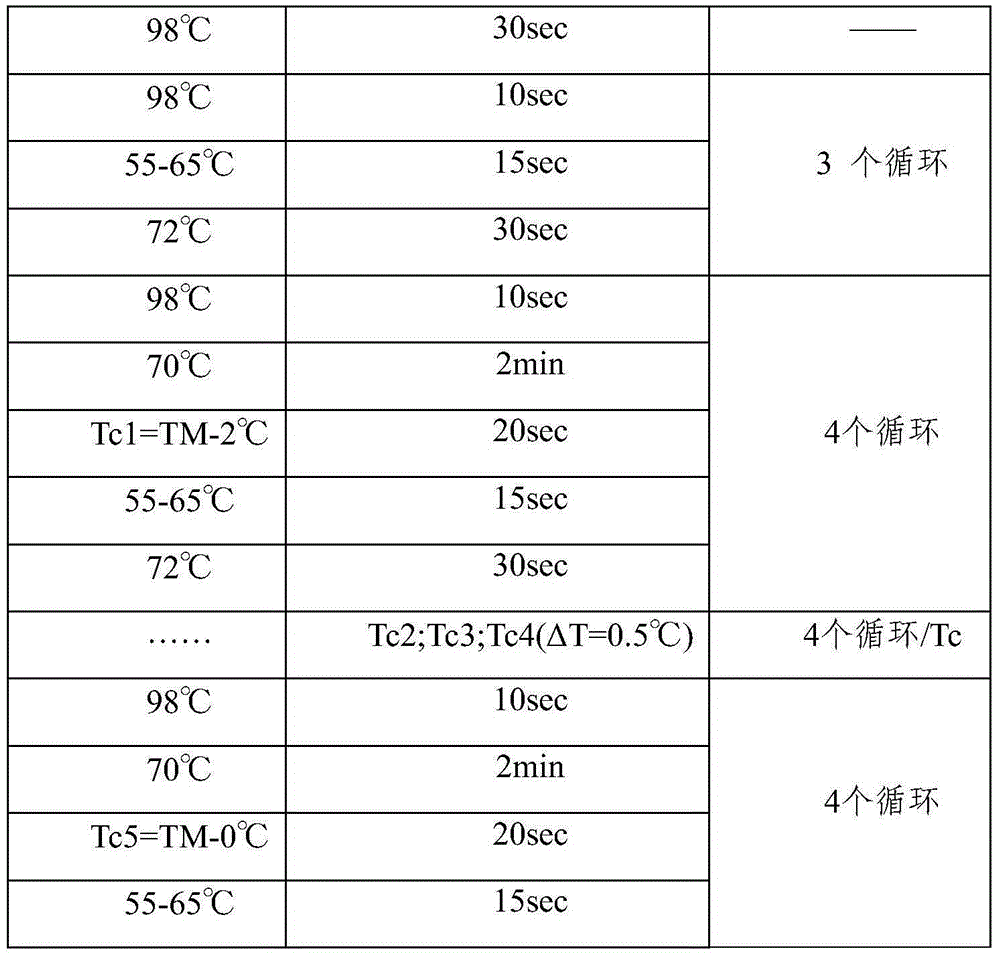
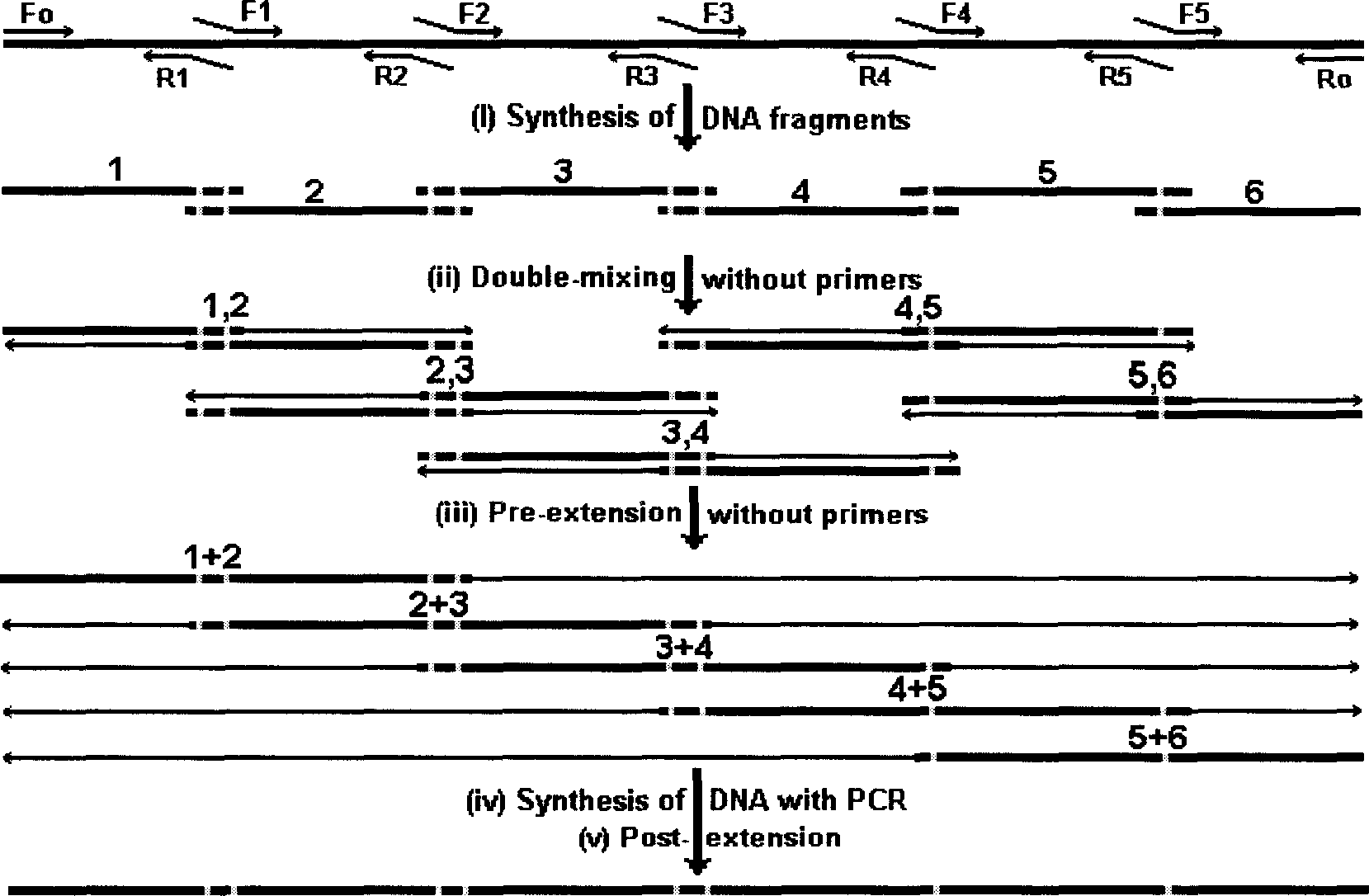
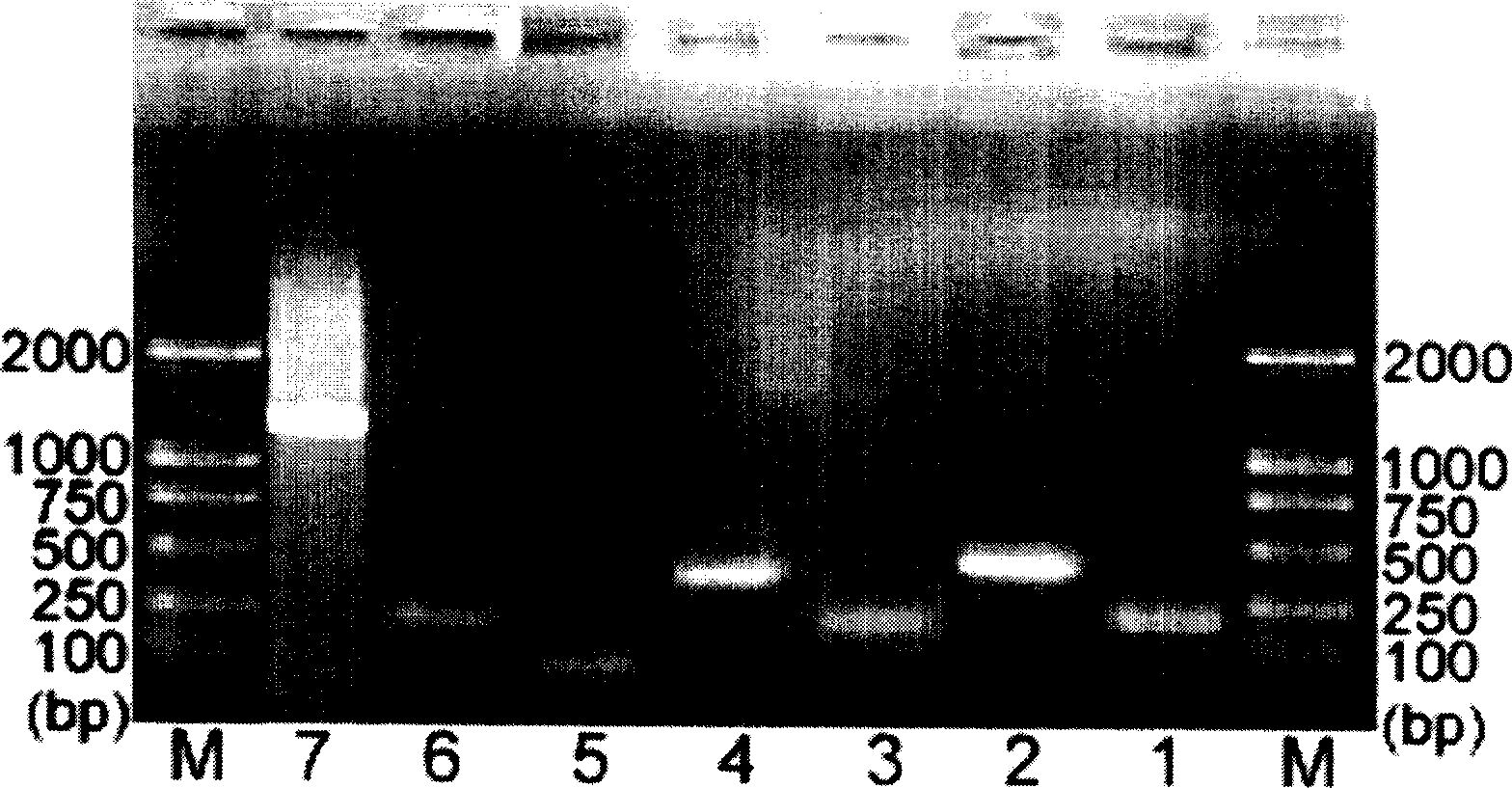
The invention relates to the method of gene fixed-point mutation which is a modified overlap extension PCR method and the mutated gene. The process includes the fragment composition, the double mixing, the prepared extension, the whole DNA composition and after extension. The method is detected by the synzyme gene sam1 from the SAM of the Saccharomyces cerevisiae and gets the mutated gene modified by the rare coden. The SAM synzyme encoding by the gene can express in the yeast and improves the yield of the intracellular SAM. The invention can reach the many fixed-point mutations in a same reaction process.
Owner:SHENYANG INST OF APPLIED ECOLOGY - CHINESE ACAD OF SCI
ARMS-PCR method for mtDNA allelic gene typing and point mutation detecting
InactiveCN101768635AEnough to accumulateReduced amplification efficiencyMicrobiological testing/measurementPcr methodPoint mutant
The invention provides an ARMS-PCR method for mtDNA allelic gene typing and point mutation detecting. When an idiosyncratic ARMS primer is in design, an upstream primer or a downstream primer is additionally provided with two continuous basic group mismatches at the 3 to 4 position counting backwards at the 3`end, the mismatching way is the exchanging of A to T or G to C, and the increasing quantity of templates mtDNA when in ARMS-PCR amplification is 2 to 10 times larger than the conventional using quantity. By improving ARMS-PCR, the invention is suitable for detection of mtDNA mutation or mtSNPs allelic gene typing. Compared with the present mtDNA pleomorphic or genovariation detecting technology, the method in the invention not only is fairly reliable in results and is simple as well as easy in operation, but also greatly quickens the analyzing process, lowers the cost and reduces the workload.
Owner:CENT SOUTH UNIV
Sarcoma fusion gene and/or mutation joint detection primer group and kit
ActiveCN112094915AAchieving Simultaneous DetectionHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationLigationTranscription (biology)
The invention relates to the technical field of molecular biology, and particularly discloses a sarcoma fusion gene joint detection primer group, kit and detection method. The kit provided by the invention comprises: 1) a special linker for DNA library construction; 2) a specific composite primer for detecting fusion genes and point mutation; and 3) a composite primer for library amplification. DNA and RNA of a to-be-detected sample are respectively extracted, reverse transcription is performed on RNA to synthesize cDNA, the cDNA is mixed with DNA, fragmentation, terminal repair and linker ligation are performed on the mixture, PCR amplification is performed on the product, a library is enriched, and high-throughput sequencing is performed. According to the fusion gene detection kit and detection method provided by the invention, various fusion genes or mutations related to sarcoma can be detected at one time, the detection efficiency is improved, the operation is convenient, the consumption time is short, and the sensitivity is relatively high and can reach 0.1%.
Owner:苏州科贝生物技术有限公司
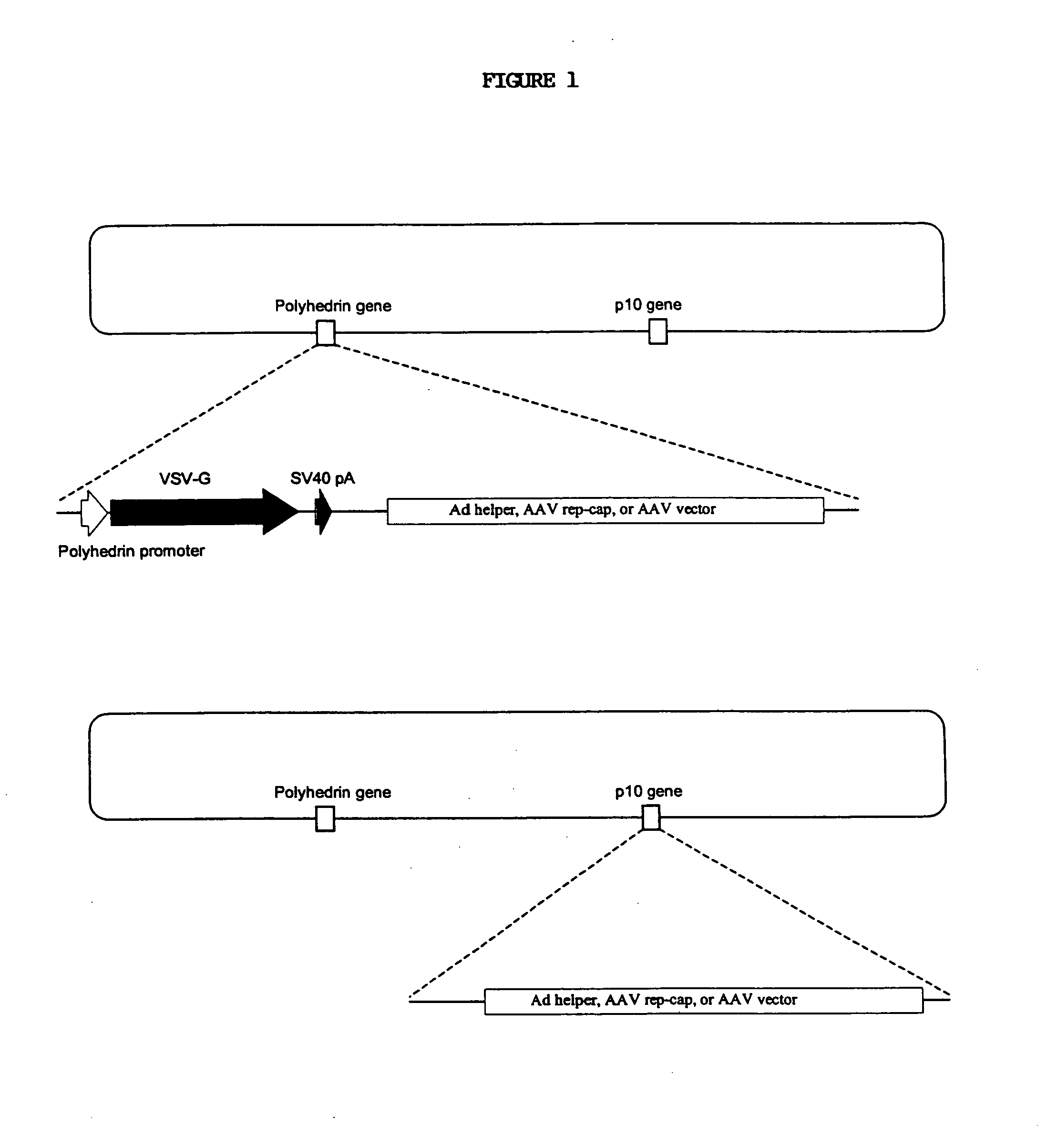
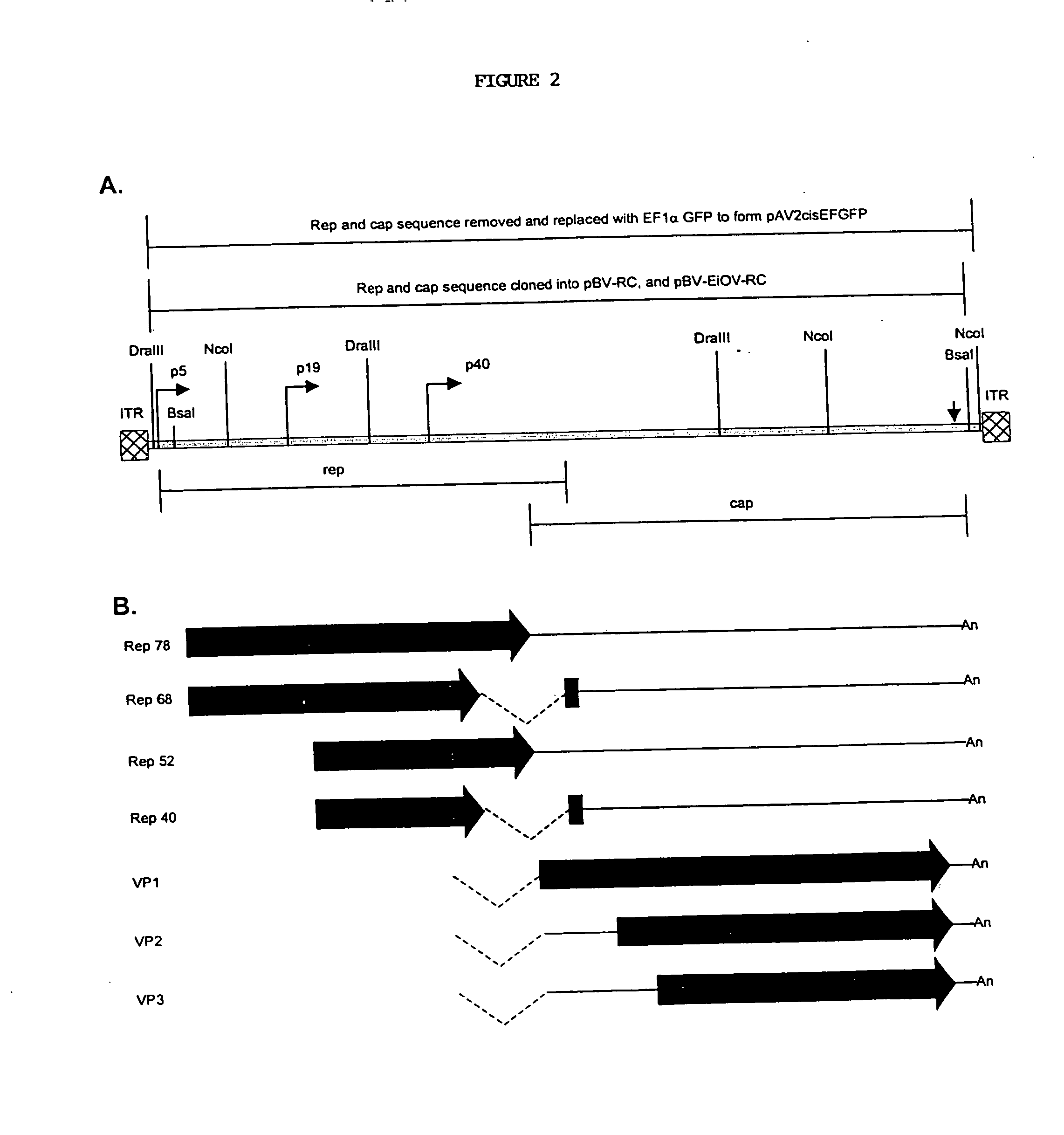
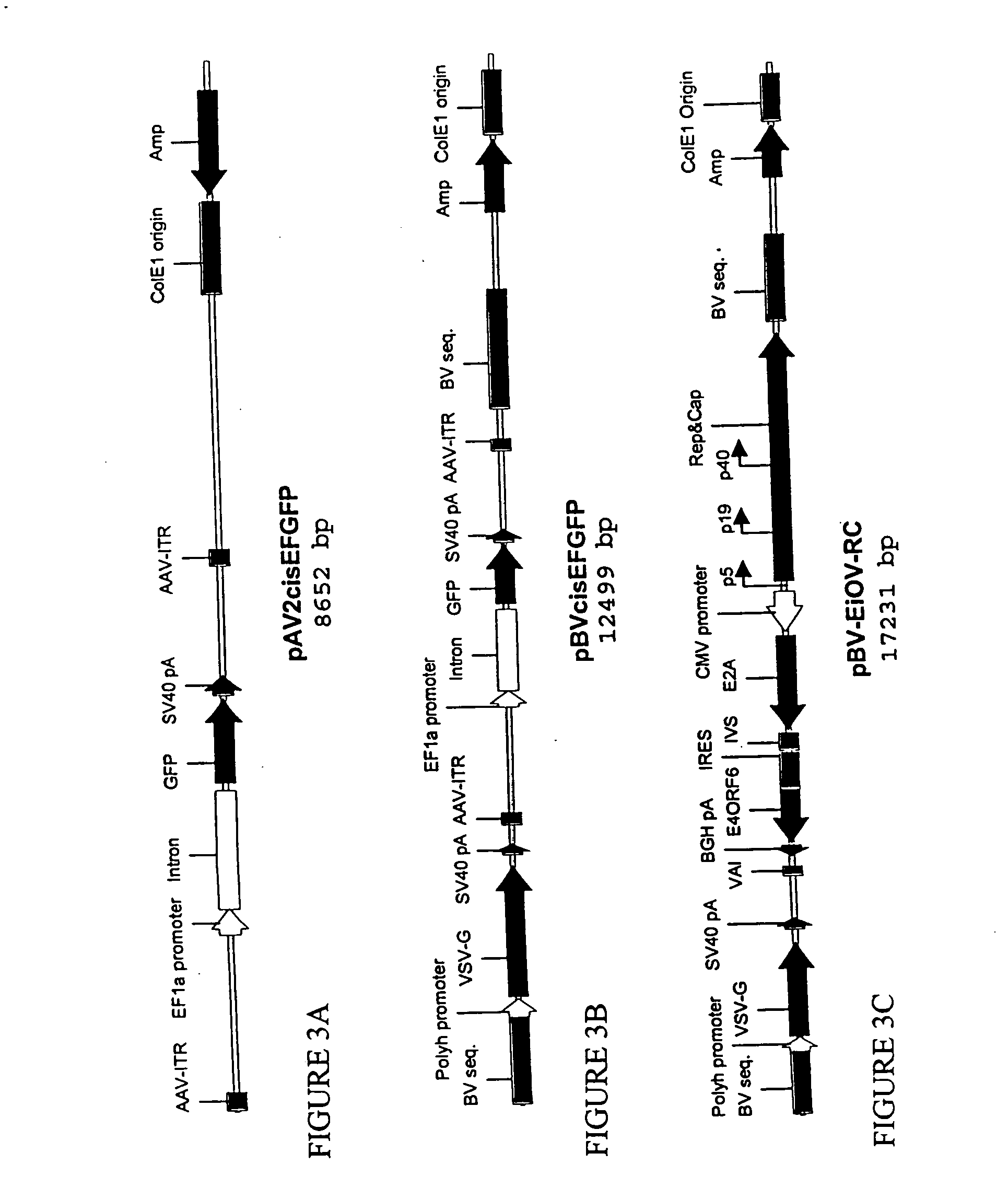
Novel compositions and methods for production of recombinant virus
This invention relates to novel nonmammalian carrier vectors and viruses useful in the production of high titers of recombinant viruses which may contain foreign DNA inserts or which may be point-mutated or deleted viruses, and methods of producing those viruses. The nonmammalian carrier vector (“carrier vector”) is a chimeric vector which includes those portions of a nonmammalian virus backbone which allow replication in a nonmammalian host cell. The carrier vector includes various nucleic acid cassettes, which may include an embedded recombinant viral genome containing a desired transgene, components necessary for production of a replication-defective recombinant virus containing the transgene, and domains that permit the carrier vector to bind to mammalian cells. The invention also provides methods of producing high concentrations of recombinant virus as a substantially homogeneous. preparation, compositions to produce the recombinant virus, and novel recombinant viruses.
Owner:GENOVO
Cyclodextrin glycosyl transferase with improved maltodextrin substrate specificity and preparation method thereof
ActiveCN102994468AIncreased substrate specificityIncrease productionBacteriaMicroorganism based processesArginineWild type
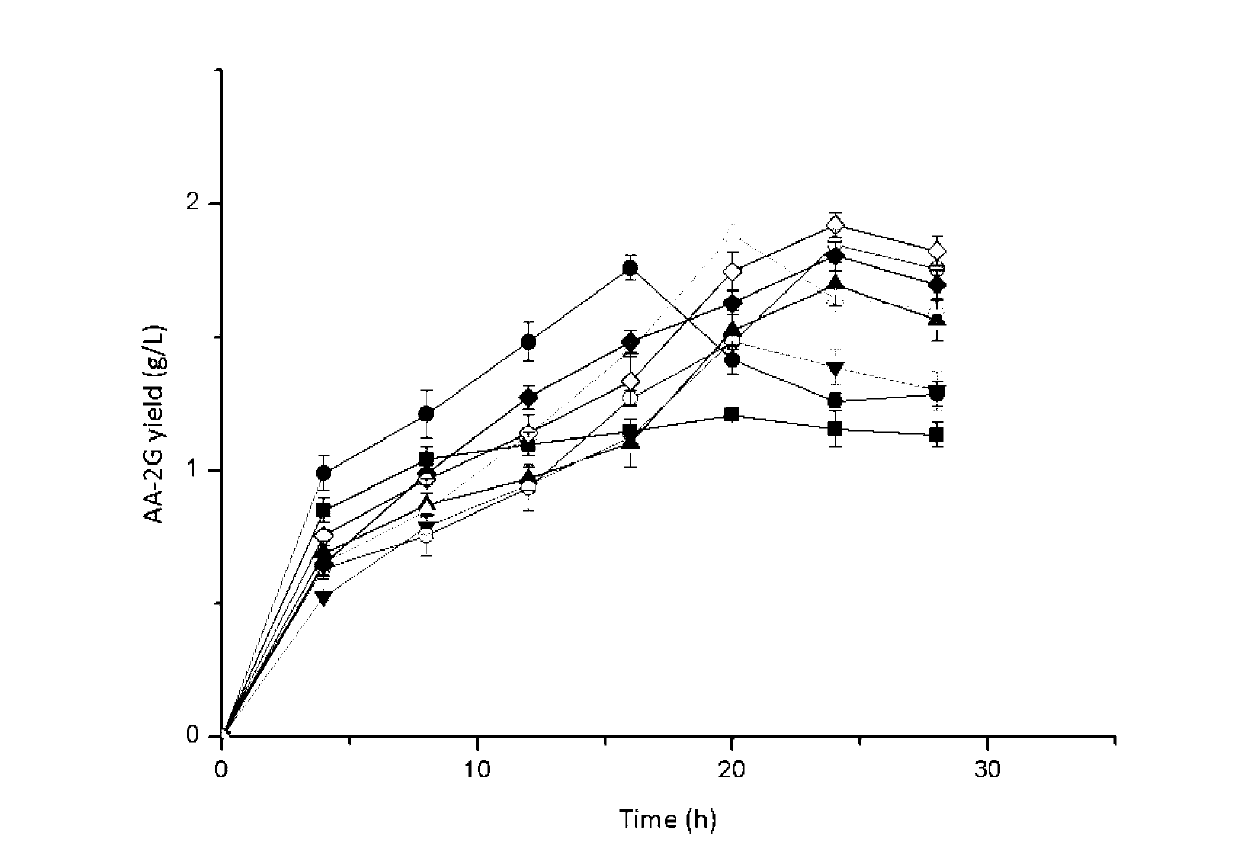
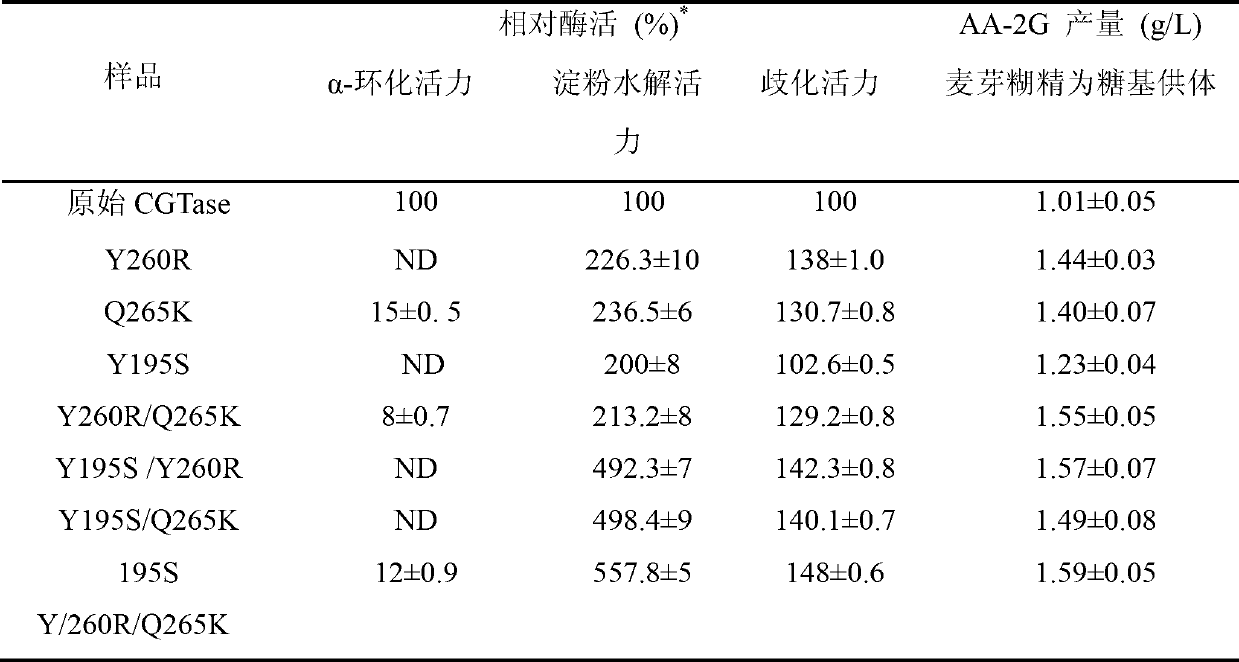
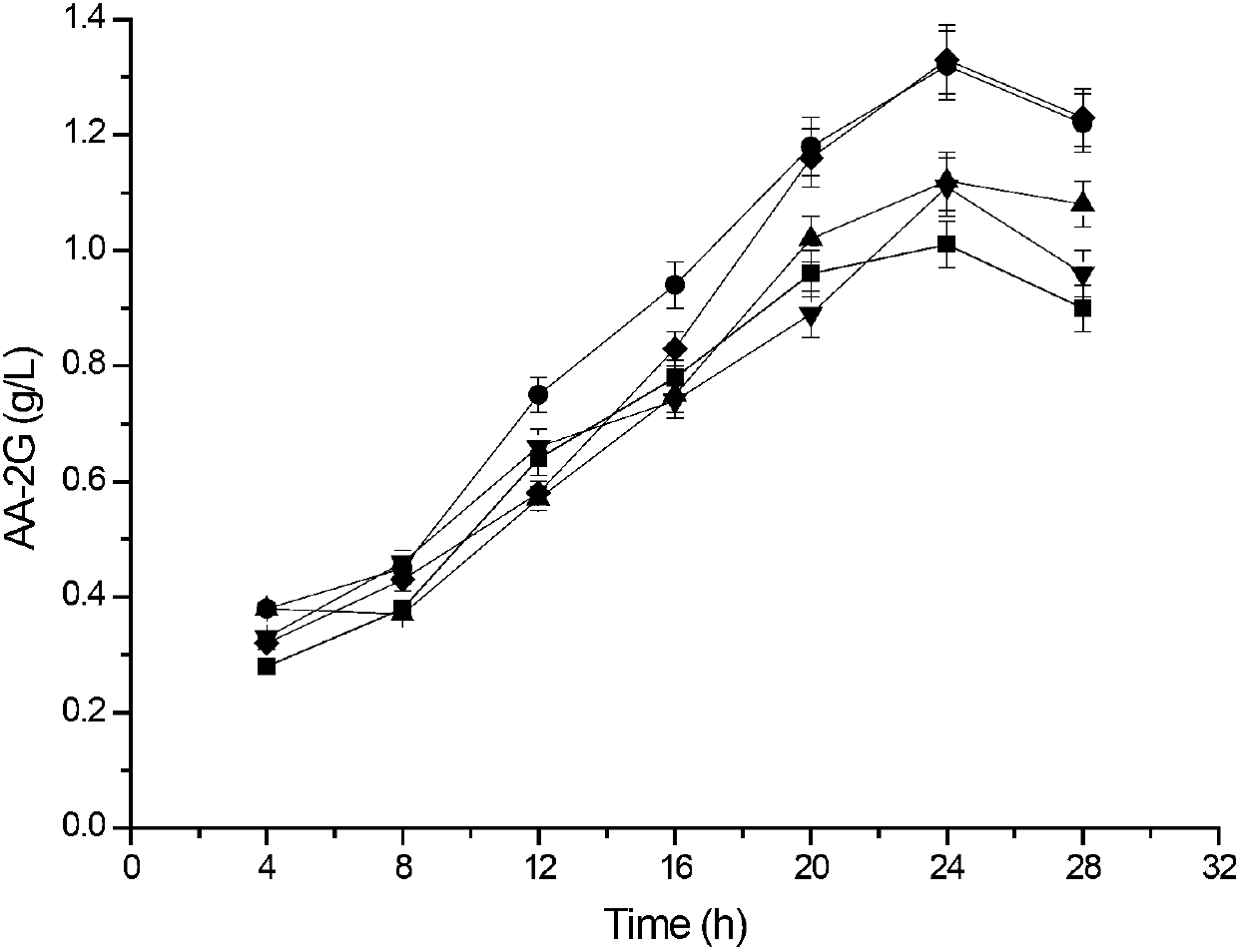
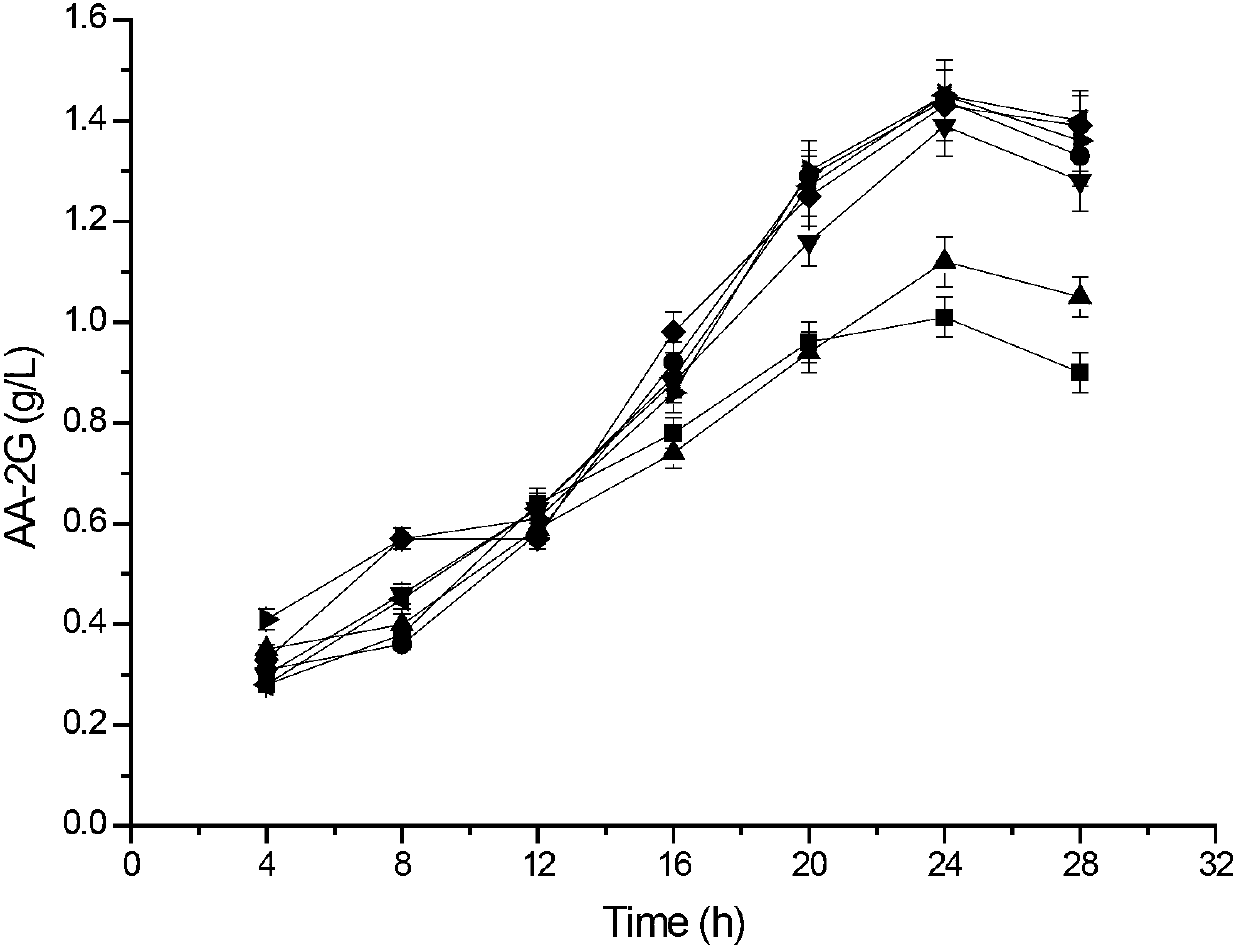
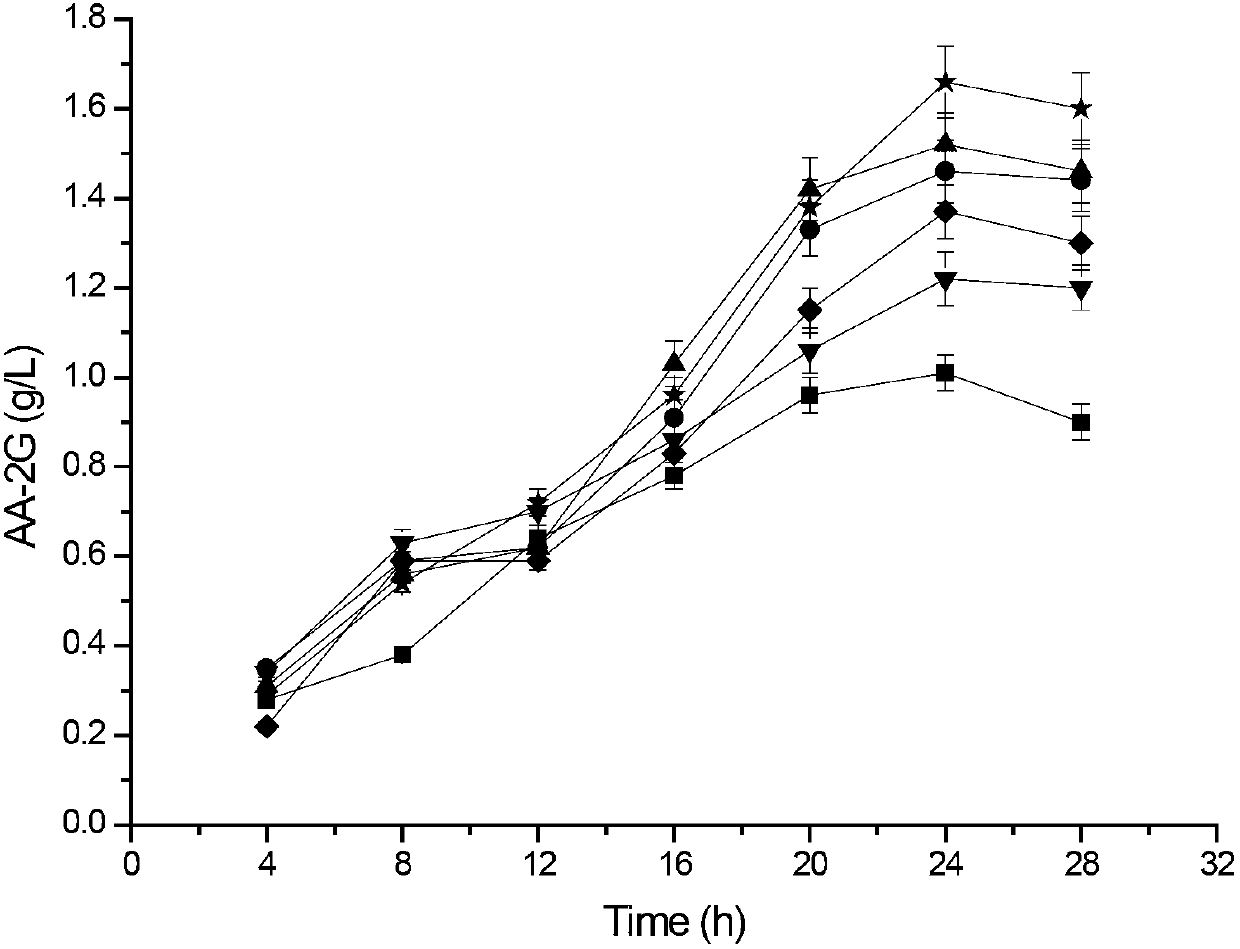
The invention discloses cyclodextrin glycosyl transferase with improved maltodextrin substrate specificity and a preparation method thereof, and belongs to the field of genetic engineering and enzyme engineering. 195-bit tyrosine (Tyr) of CGTase of P.macerans strain JFB05-01 (CCTCC NO:M208063) is replaced by serine (Ser), 260-bit tyrosine (Tyr) is replaced by arginine (Arg), and 265-bit glutamine (Gln) is replaced by lysine (Lys), so that the AA-2G yield is respectively improved by 23 percent, 44 percent and 40 percent. According to combined mutation of the mutant strains, double mutants Y195S / Y260R, Y195S / Q265K and Y260R / Q265K and A three-point mutant Y195S / Y260R / Q265K are obtained. A Glycosyl donor is produced by the maltodextrin, so that the AA-2G yield is respectively improved by 57 percent, 49 percent, 55 percent and 59 percent; and compared with the mild type CGTase, the mutant strains facilitate the production of the AA-2G for glycosyl donors by using maltodextrin.
Owner:JIANGNAN UNIV
Cyclodextrin glycosyl transferase with improved maltodextrin substrate specificity and preparation method thereof
InactiveCN103122341AIncreased substrate specificityIncrease productionBacteriaTransferasesCyclodextrinWild type
The invention discloses a cyclodextrin glycosyl transferase with improved maltodextrin substrate specificity and a preparation method thereof and belongs to the fields of genetic engineering and enzyme engineering. According to the invention, for CGTase derived from peanibacillus macerans, lysine at a 47th site, tyrosine at a 89th site, asparaginate at a 94th site and aspartic acid at a 196th site are respectively mutated into leucine K47L, phenylalanine Y89F, praline N94P and tyrosine D196Y, so that AA-2G yields are respectively increased by 30.7%, 10.9%, 10.0% and 31.7%. Complex mutation is carried out on the mutant strains to obtain double mutants namely K47L / Y89F, K47L / N94P, K47L / D196Y, Y89F / N94P, Y89F / D196Y and N94P / D196Y, three-point mutants namely K47L / Y89F / N94P, K47L / Y89F / D196Y, K47L / N94P / D196Y and Y89F / N94P / D196Y and a four-point mutant namely K47L / Y89F / N94P / D196Y. Yields of AA-2G produced by the mutants while maltodextrin is utilized as a glycosyl donor are respectively increased by 42.6%, 11.0%, 37.6%, 43.6%, 43.6%, 41.6%, 44.5%, 50.5%, 20.8%, 35.6% and 64.4%. Compared with the wild type CGTase, the mutants is more beneficial to production of the AA-2G while the maltodextrin is utilized as the glycosyl donor.
Owner:JIANGNAN UNIV
Cell strain transfacted by delection/Point mutont gene using histo type cellulolytic proenzyme as activator
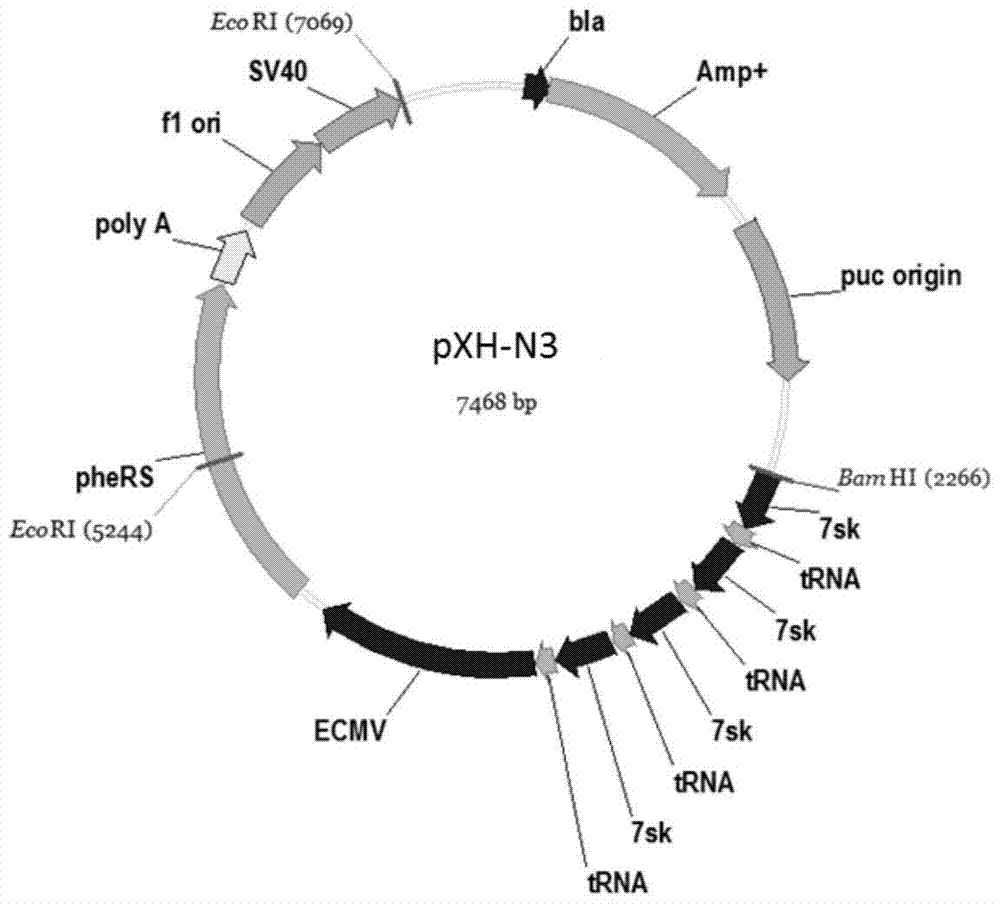
The invention is a tissue type plasminogen activator mutant rPA(K) gene transfected cell strain, using liposome mediated transformation to transfect the Eukaryotic expression carrier pCSRK of the composed t-PA Cdna deleted / point mutant rPA(K)-rPA gene (KHRR296-299AAAA) (this mutant can escape the inhibition by PAI-1) with CHO-dhfr-, and by double selection of G418 and DMEM, obtaining its stable expression cell strain CHO-dhfr-pCSRK (conservation number: CGMCC No.1094).
Owner:NINGXIA UNIVERSITY
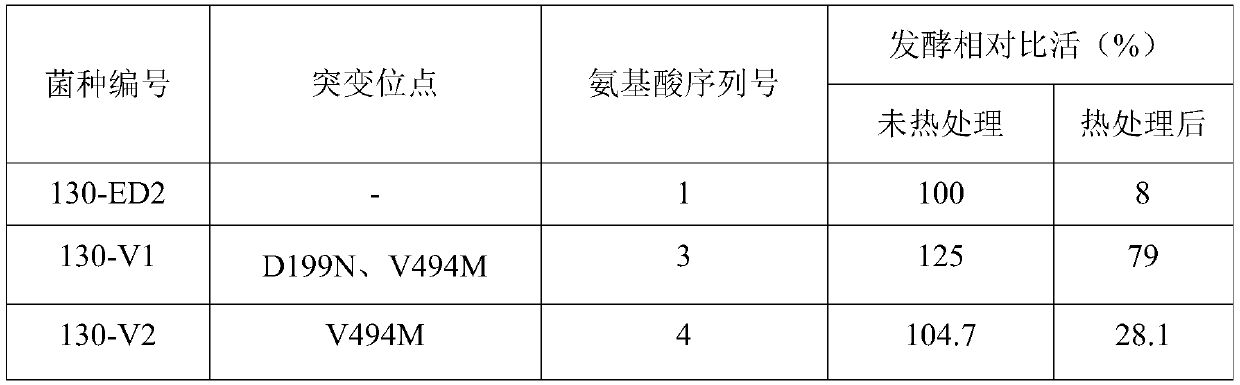
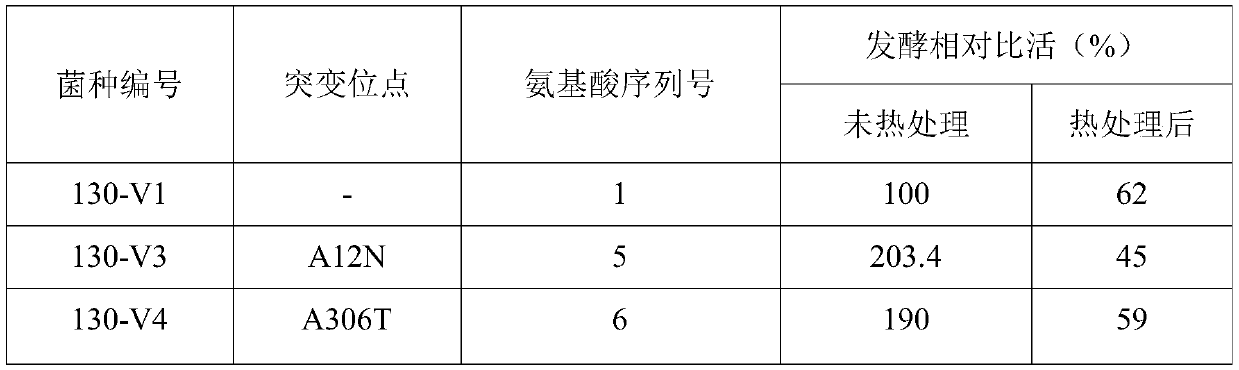
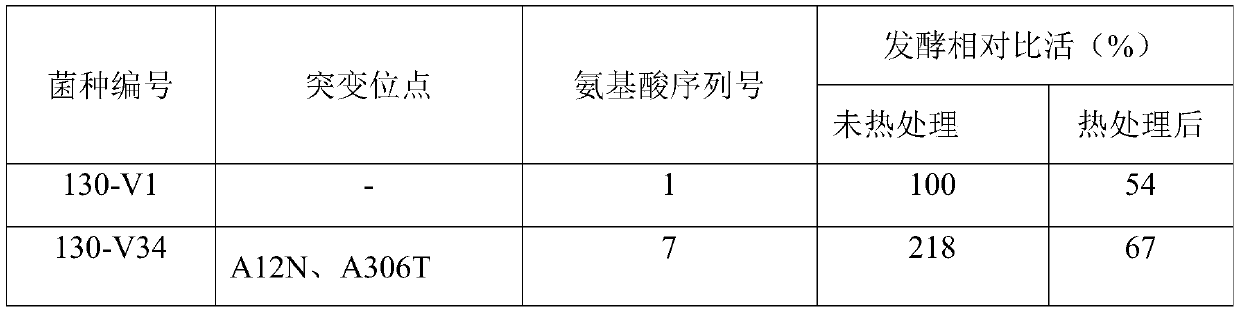
Cephalosporin C acylase mutant with high thermal stability
ActiveCN111172142AImprove thermal stabilityIncrease enzyme activityFungiBacteriaAklanonic acidPoint mutant
The invention discloses a cephalosporin C acylase mutant with a high thermal stability constructed by point mutation, which has an amino acid sequence selected from SEQ ID Nos: 3-7. Compared with initial cephalosporin C acylase with SEQ ID No: 1, the cephalosporin C acylase mutant disclosed by the invention has high thermal stability and higher enzyme activity, so that the cephalosporin C acylasemutant can be used for producing 7-aminocephalosporanic acid by adopting an one-step enzymatic method.
Owner:SHANGHAI TAOYUSHENG BIOTECHNOLOGY CO LTD
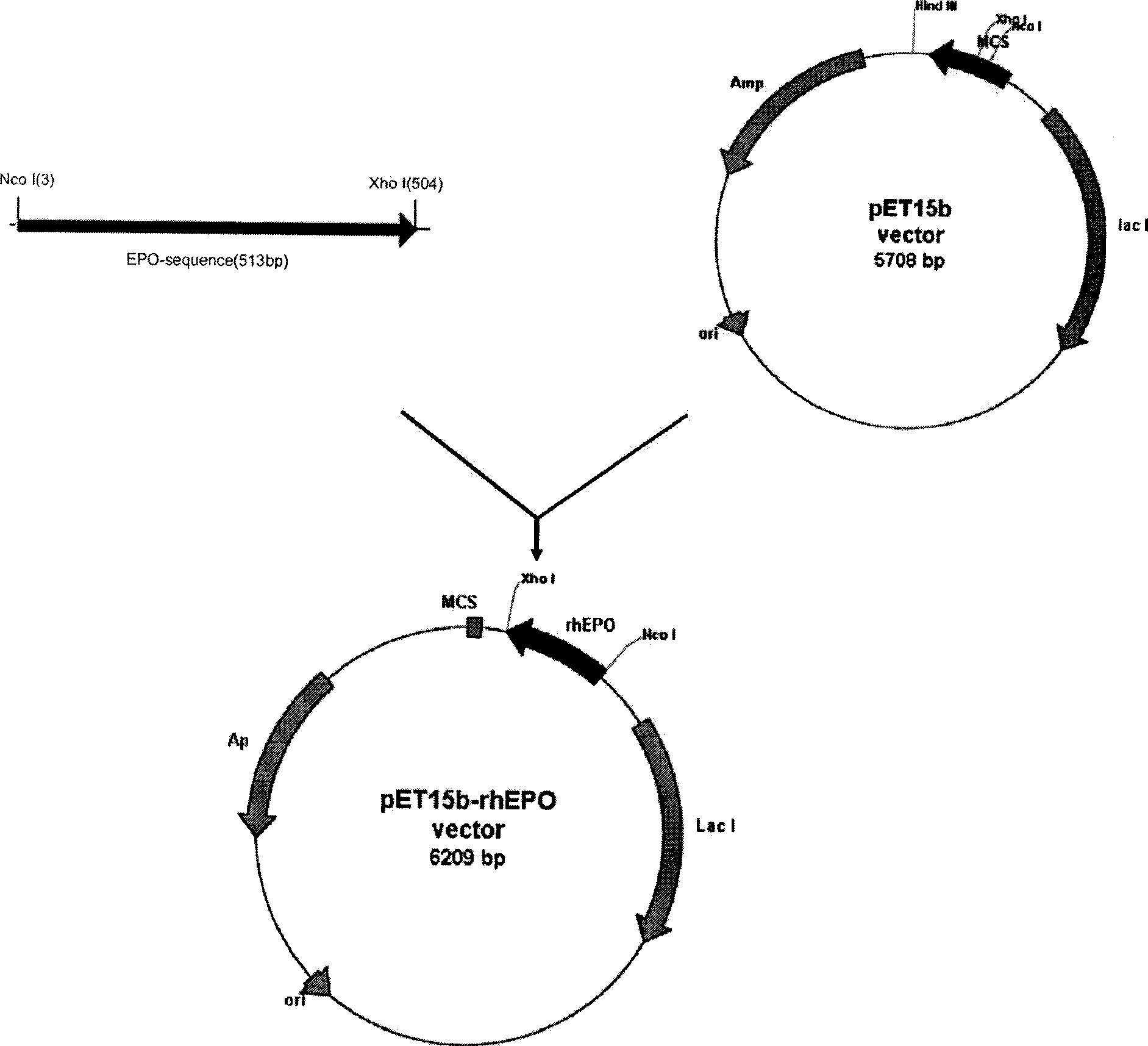
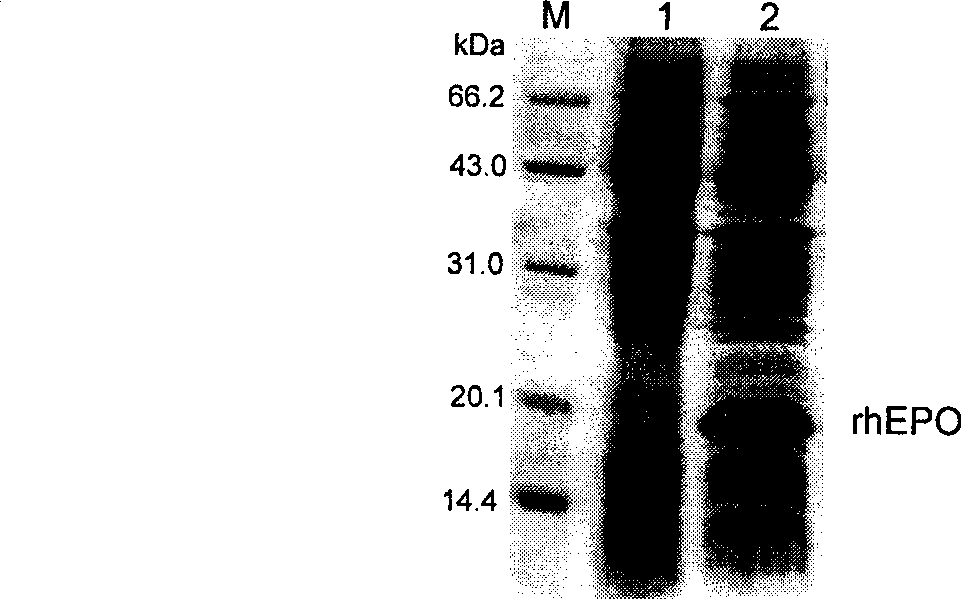
PEGylated erythropoietin protein long-acting preparation
InactiveCN101376676AImprove convenienceGood curative effectPeptide/protein ingredientsErythropoietinAIDS chemotherapyPegylated erythropoietin
The invention discloses a type of polyethylene glycol erythropoietin, which is prepared from non-glycosylated rhEPO(rhngEPO) or point mutant thereof, and N-terminal amino of single and single chain PEG molecule having molecular weight greater than 20kDa or mercapto of cysteine mutational state by fixed point coupling, wherein the olyethylene glycol erythropoietin has the molecular structural formula of CH3-(CH2CH2-O)n-(CH2)r-NH-rhngEPO or CH3-(CH2CH2-O)n-X-S-rhngEPOm, wherein n is 100 to 2,200, r is 0 to 4, and X is S or C. The PEG-rhngEPO or PEG-rhEPOm molecule simultaneously has long-acting preparation characteristics such as long acting, high efficiency and low immunogenicity, and establishes foundation for preparing parent long-acting preparation for treating anemia caused by chronic renal insufficiency, tumor, anemia caused by AIDS chemotherapy, marrow brain nerve damage, and selective operation auto-transfusion.
Owner:TIANJIN PAIGE BIOTECHNOLOGY CO LTD
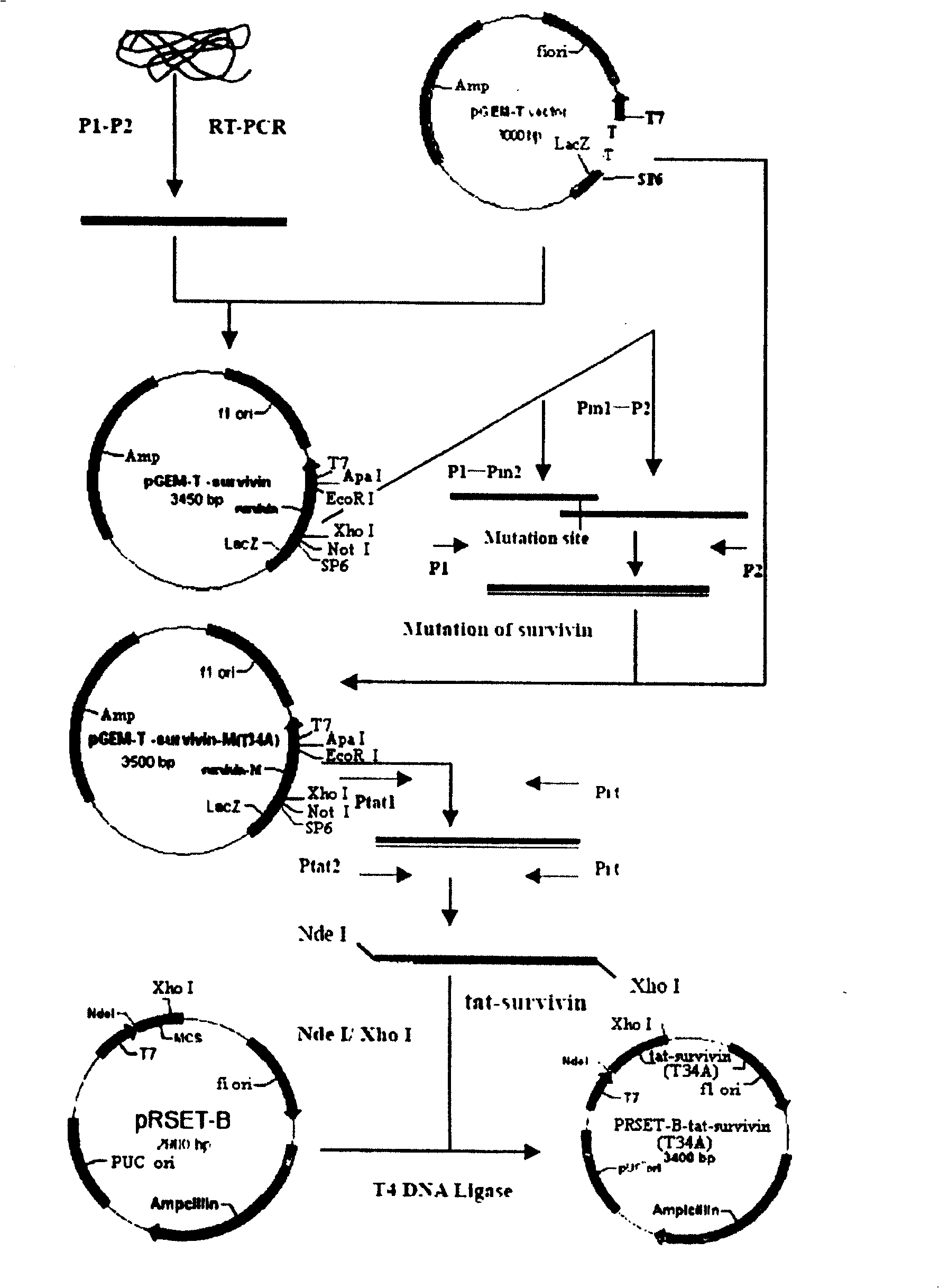
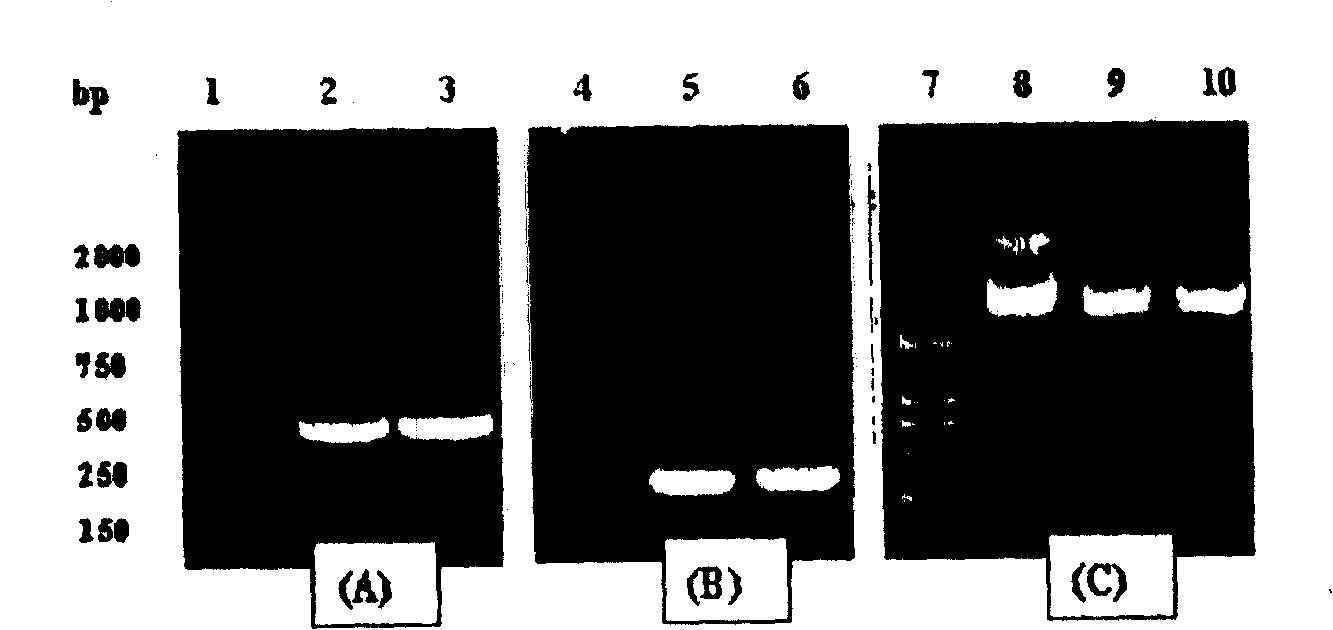
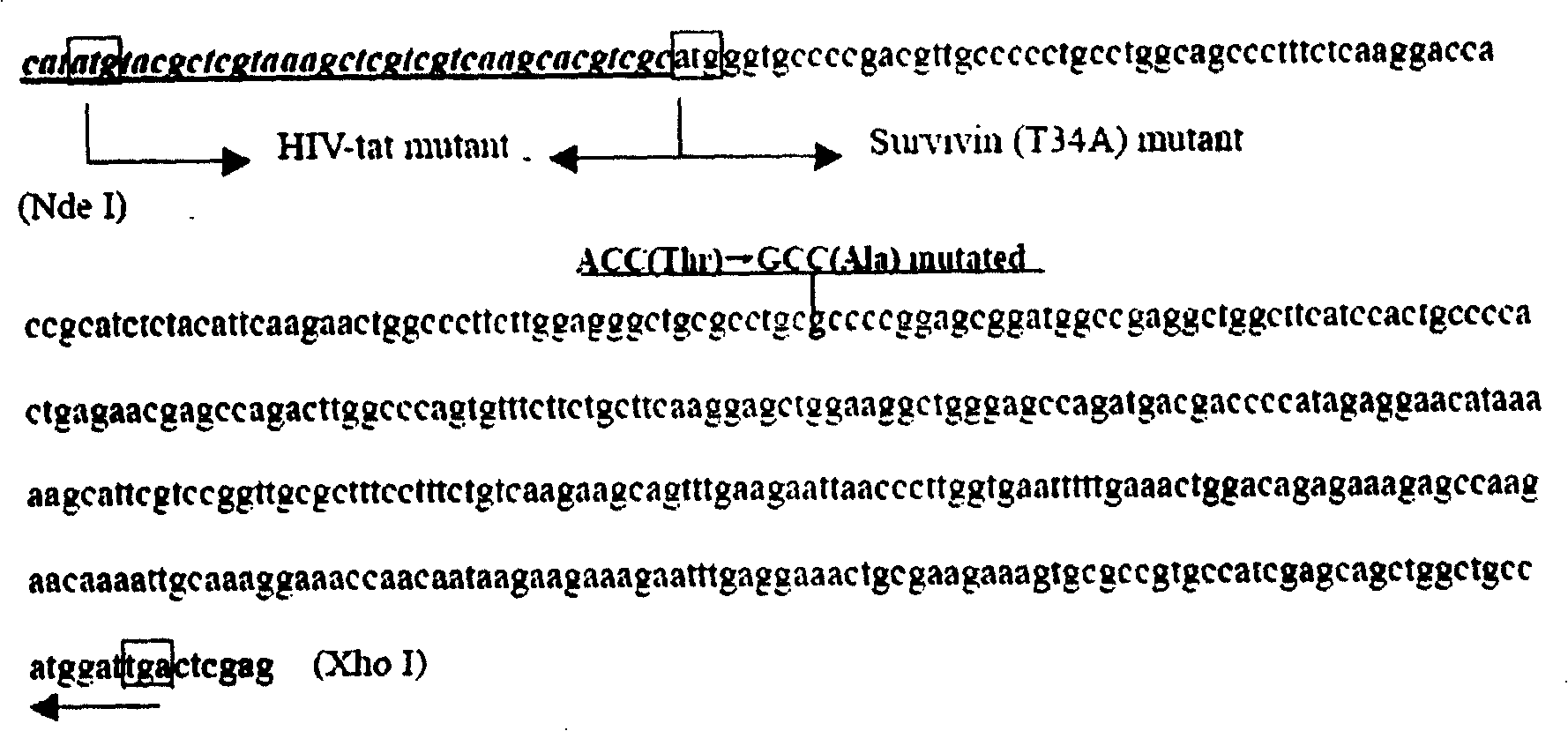
Survivin mutant containing HIV transduction structural area and its preparation method and uses
InactiveCN100424096CPrevent genetic mutationStrong inhibitory activityPeptide/protein ingredientsHybrid peptidesEscherichia coliFhit gene
Owner:EAST CHINA UNIV OF SCI & TECH
DNA sequence, and recombinant preparation of the grass pollen allergen Lol p4
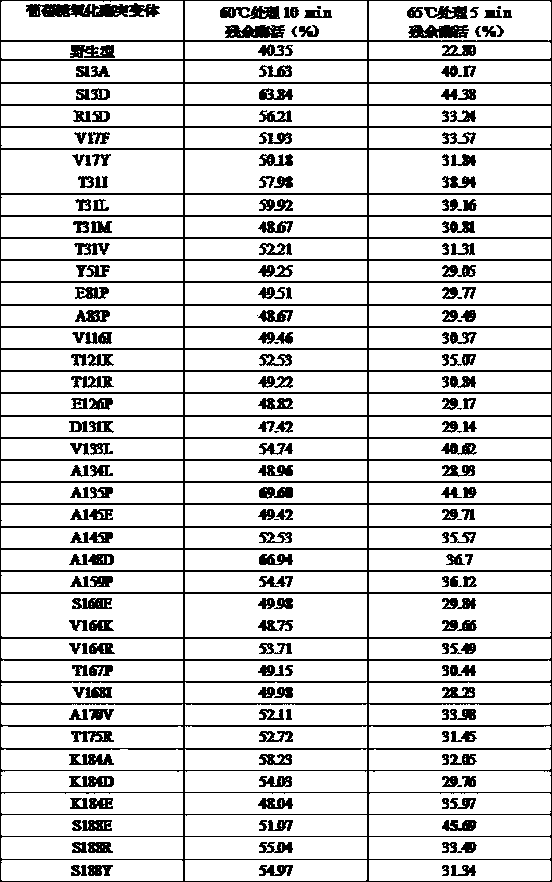
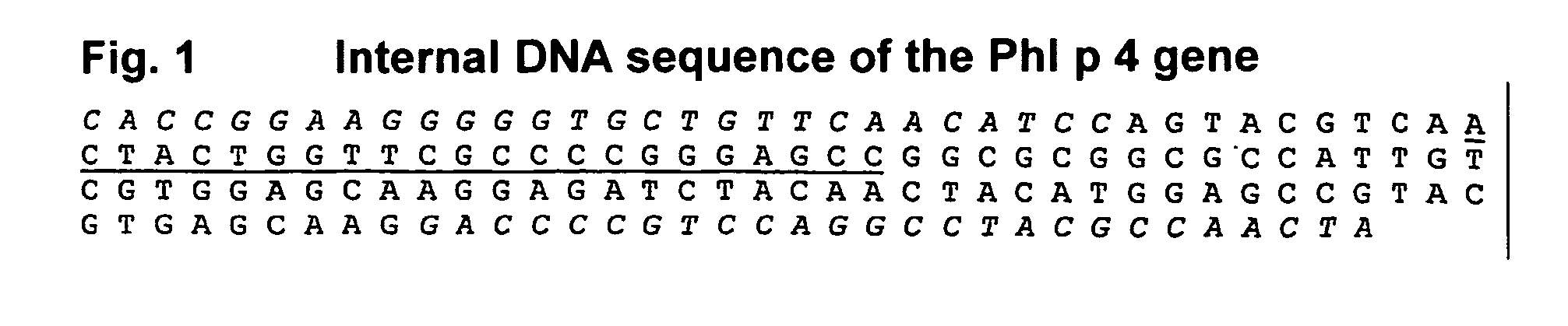
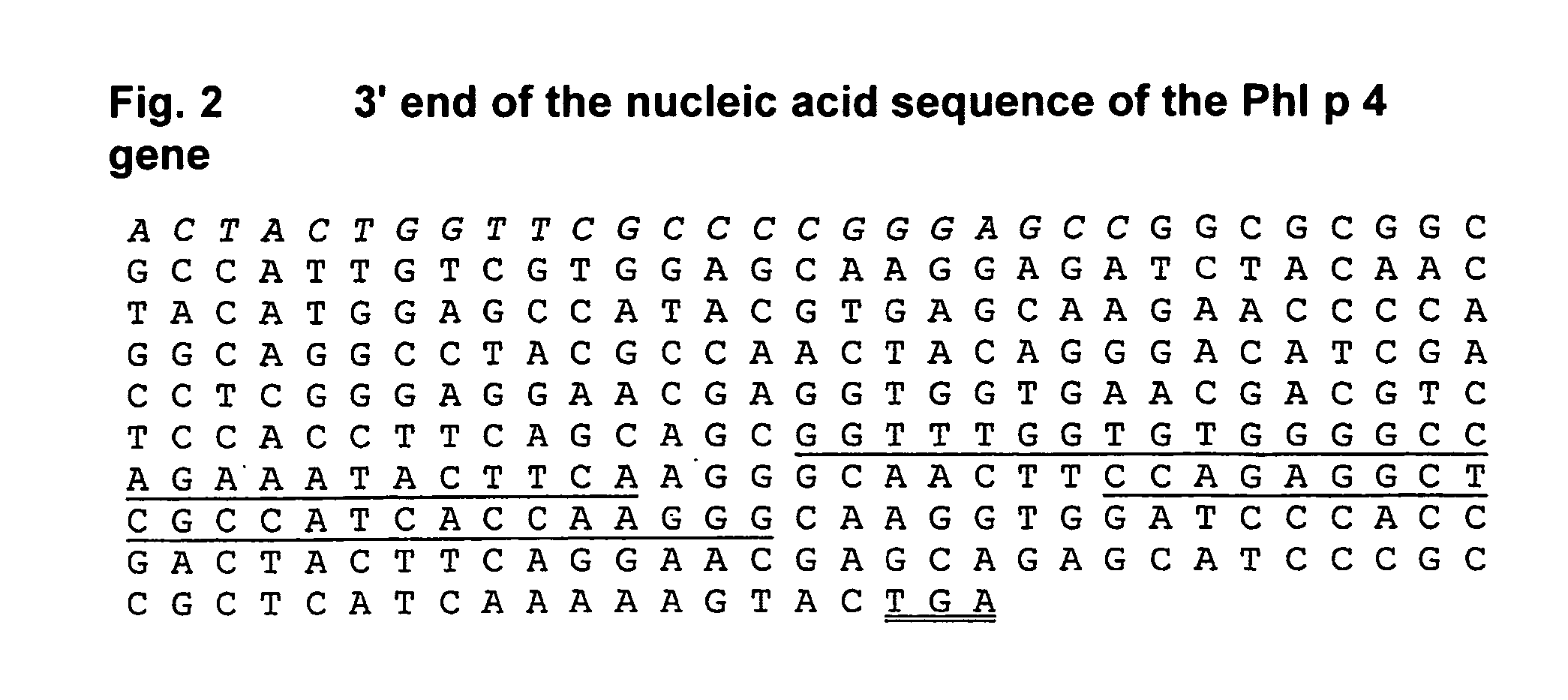
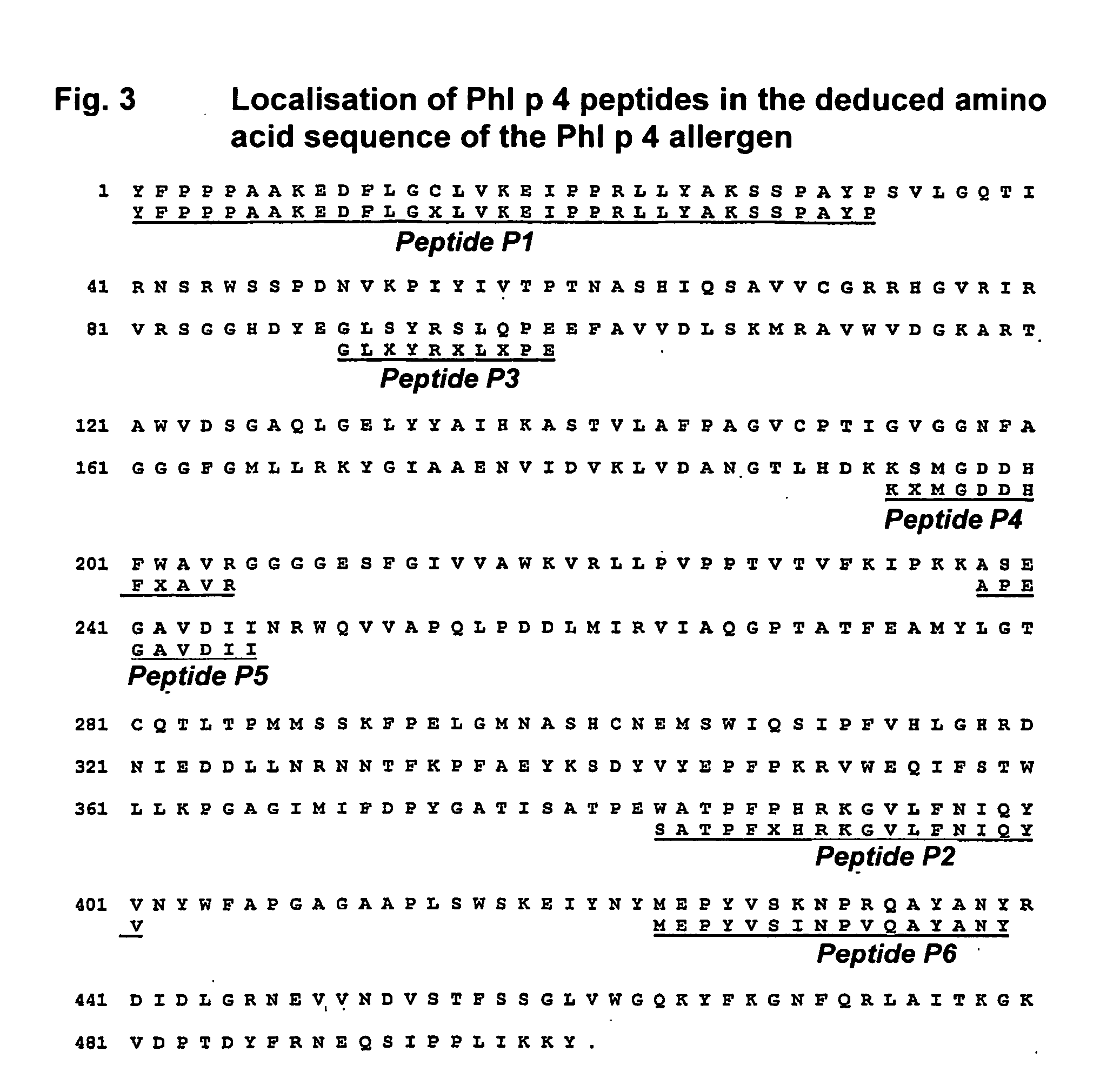
The present invention relates to the provision of a DNA sequence of the major grass pollen allergen Lol p 4. The invention also encompasses fragments, new combinations of partial sequences and point mutants having a hypoallergenic action. The recombinant DNA molecules and the derived polypeptides, fragments, new combinations of partial sequences and variants can be utilized for the therapy of pollen-allergic diseases. The proteins prepared by recombinant methods can be employed for in vitro and in vivo diagnosis of pollen allergies.
Owner:MERCK PATENT GMBH
Lovastatin acyltransferase containing one or more point mutations
ActiveCN104342416AIncrease profitSimple post-processingFungiTransferasesAmino acid mutationPoint mutant
The invention relates to a lovastatin acyltransferase mutant. The mutant contains an acyltransferase amino acid sequence represented by SEQIDNO.1 of wild A. terreus and at least 2-6 amino acid mutations selected from N45R, A110P, S145N, Q230M, L293M and L379M. The invention also relates to a nucleic acid nucleic acid for coding the mutant, a relevant expression vector, a host cell, and a use of the mutant in the synthesis of lovastatin.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Plasma free DNA lung cancer gene combined detection kit
ActiveCN112094916AAchieving Simultaneous DetectionHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationMedicineHigh throughput sequence
The invention relates to the technical field of molecular biology, and particularly discloses a plasma free DNA lung cancer fusion gene combined detection primer group and kit. The kit provided by theinvention comprises: 1) a special linker for DNA library construction; 2) a specific composite primer for detecting fusion genes and point mutation; and 3) a composite primer for library amplification. According to the detection kit, DNA of a sample to be detected is extracted and subjected to fragmentation, terminal repair and linker connection, a product is subjected to PCR amplification, and high-throughput sequencing is performed after library enrichment. According to the fusion gene detection kit and detection method provided by the invention, various fusion genes or mutations related tolung cancer can be detected at one time, the detection efficiency is improved, the operation is convenient, the consumption time is short, and the sensitivity is relatively high and can reach 0.0625%.
Owner:苏州科贝生物技术有限公司
Novel glucose oxidase mutant
ActiveCN108118036AImprove heat resistanceFungiMicroorganism based processesAfter treatmentHeat resistance
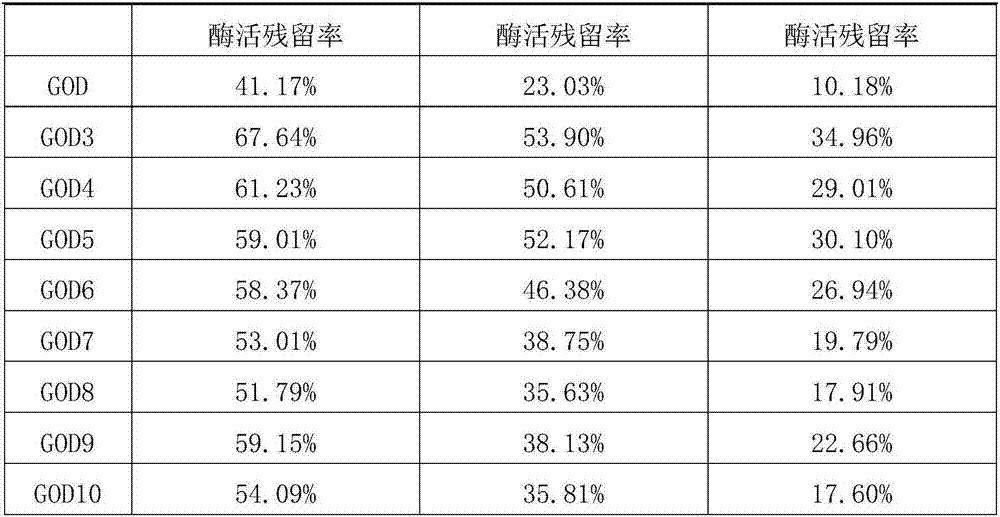
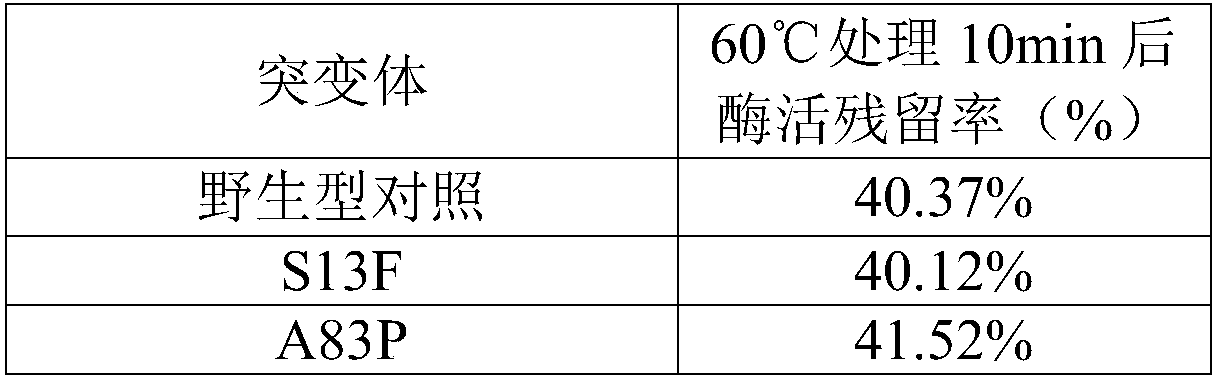
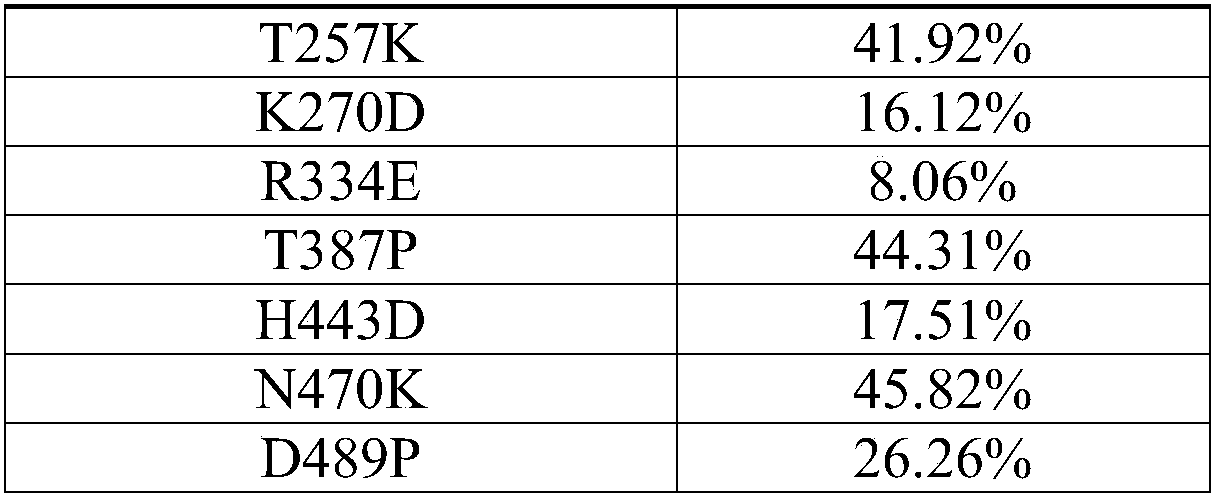
The invention relates to the technical field of genetic engineering, and specifically provides a glucose oxidase mutant. According to the present invention, the heat resistance of the glucose oxidasesingle-point mutant is generally higher than the heat resistance of wild type glucose oxidase, the residual enzyme activity of the glucose oxidase single-point mutant is increased by 17.5-72.7% aftertreatment for 10 min at a temperature of 60 DEGC, and the residual enzyme activity of the glucose oxidase single-point mutant is increased by 23.8-100.4% after treatment for 5 min at a temperature of65 DEGC, such that the heat resistance of glucose oxidase is substantially improved due to the single-point mutation, the glucose oxidase single-point mutant can be well used as the feed additive compared to the wild type glucose oxidase, and the wide applications of glucose oxidase in feed can be easily achieved so as to provide wide market prospect.
Owner:QINGDAO VLAND BIOTECH GRP
Dna sequence and recombinant production of the grass pollen allergen phl p4
The present invention relates to the provision of the genetic sequence of the major grass pollen allergen Phl p 4. The invention also covers fragments, new combinations of partial sequences and point mutants having a hypoallergenic action. The recombinant DNA molecules and the derived polypeptides, fragments, new combinations of partial sequences and variants can be utilised for the therapy of pollen-allergic diseases. The proteins prepared by recombinant methods can be employed for the in vitro and in vivo diagnosis of pollen allergies.
Owner:MERCK PATENT GMBH
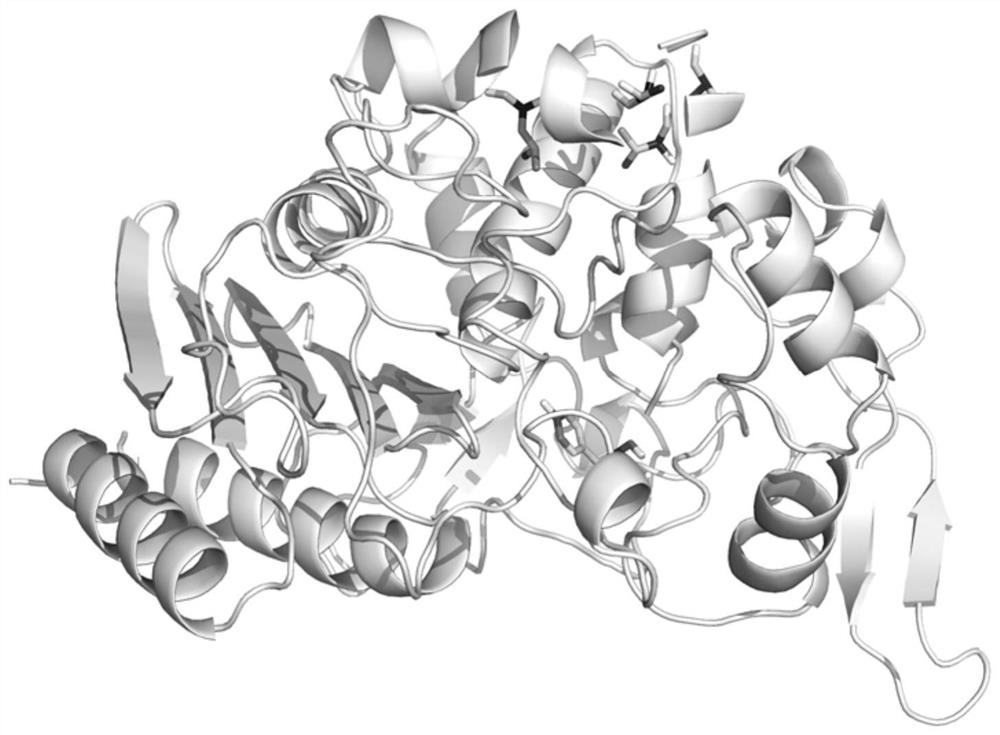
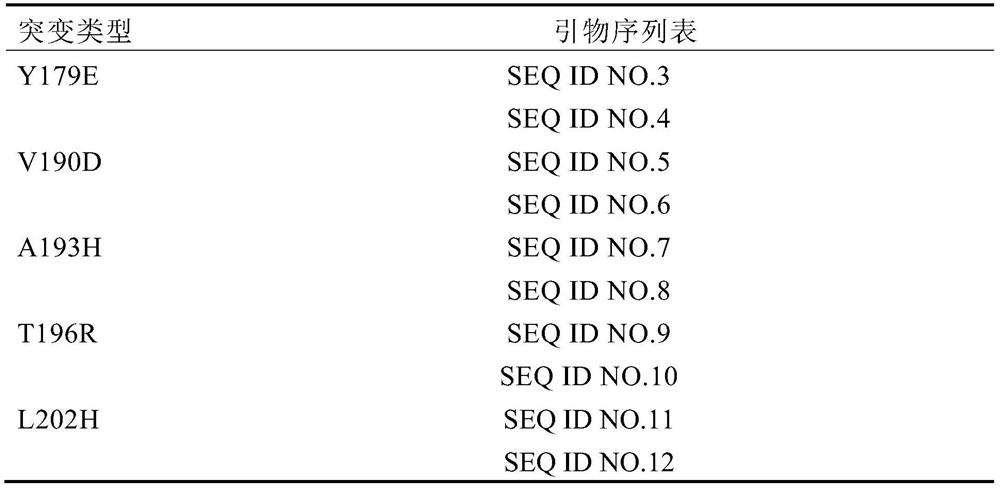
EstWY enzyme mutant having improved activity
The invention discloses an EstWY enzyme mutant having improved activity, and belongs to the technical field of enzyme engineering. An EstWY enzyme sequence is analyzed by using a consensus method without phylogenetic prejudice to obtain single-point mutants having remarkably improved activity through screening, and site-specific mutagenesis is performed on the single-point mutants to obtain mutantY179E / V190D, T196R / L202H and V190D / A193H / T196R enzymes having improved activity, which are 3 times higher than wild enzymes. The EstWY enzyme mutant provided by the invention is better in activity and shows excellent catalytic activity when creatine is catalyzed to generate fatty acids and alcohols at a relatively high temperature, besides, the constructed EstWY enzyme genetically engineered bacterium can efficiently express the EstWY enzyme mutant, and the EstWY enzyme genetically engineered bacterium has the advantages of simple culture conditions, short culture period, convenience in purification of expression products and the like.
Owner:上海绅道生物科技有限公司
Variant of Kex2 enzyme and stable expression method
InactiveCN110982808AHigh target proteinIncrease enzyme activityFungiMicroorganism based processesProtein targetSecretion expression
The invention discloses a variant of Kex2 enzyme and a stable expression method, the variant is formed by properly truncating a Kex2 amino acid sequence and performing point mutation, the variant canstably and efficiently secrete and express a target protein through a constitutive pichia pastoris expression system, and good biological enzyme activity is maintained. The fermentation process is simple, efficient, low in cost, high in product activity and suitable for commercial production.
Owner:VONSUN PHARMATECH CO LTD
Glucose oxidase mutant
ActiveCN107988177AImprove heat resistanceAccessory food factorsOxidoreductasesEpitopeAfter treatment
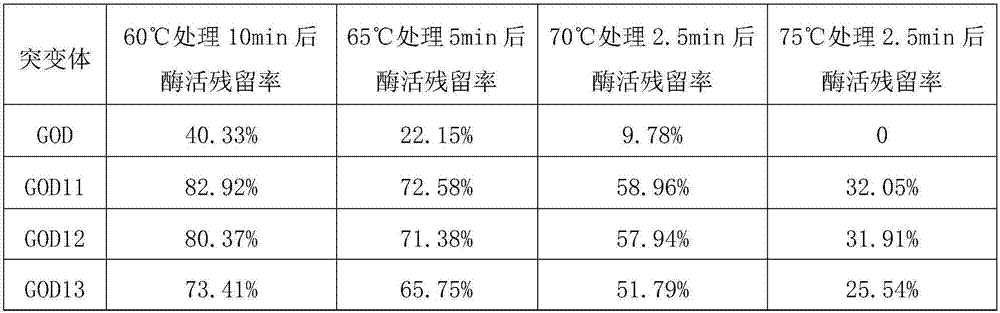
The invention aims to provide a glucose oxidase mutant. The glucose oxidase mutant contains (I) a sequence which is at least 70% homologous with the amino acid sequence, shown as SEQ ID NO:1, of glucose oxidase, as well as (II) a sequence containing at least one immune epitope of the glucose oxidase in (I),and the amino acid sequence of the glucose oxidase is obtained by modification, substitution, deletion or addition of one or more amino acids, and substitution refers to substitution for one or two amino acids. The heat resistance of the single-point mutant of the glucose oxidase is generally higher than that of wild glucose oxidase, the heat resistance of a two-point mutant is further improved as compared with the corresponding single-point mutant, residual enzyme activity after treatment at 60 DEG C for 10 min reaches 71.19%-82.92%, residual enzyme activity after treatment at 65 DEG C for 5 min reaches 63.03%-72.58%, residual enzyme activity after treatment at 70 DEG C for 2.5 minreaches 49.91%-58.96%, and residual enzyme activity after treatment at 75 DEG C for 2.5 min reaches 24.78%-32.05%.
Owner:WEIFANG KANGDIEN BIOTECH +2
Glucose oxidase mutant
ActiveCN109207446AImprove heat resistanceAccessory food factorsOxidoreductasesAfter treatmentBiochemistry
The invention relates to the technical field of protein engineering, in particular to a glucose oxidase mutant. The glucose oxidase three-point mutant GOD-K has residual enzyme activity of 68.20% after processing at 70 DGE C for 2.5 min, after treatment at 75 DEG C for 2.5 min, the residual enzyme activity is 42.76%, which are 19.3% and 38.1% higher than that of the two point mutant GOD11, respectively. The results showed that the mutant GOD11 had unexpected effect and was more suitable for application in industrial production, so its market prospect was broad.
Owner:QINGDAO VLAND BIOTECH GRP
Glucose oxidase mutant
ActiveCN109423483AImprove heat resistanceAccessory food factorsOxidoreductasesHeat resistanceWild type
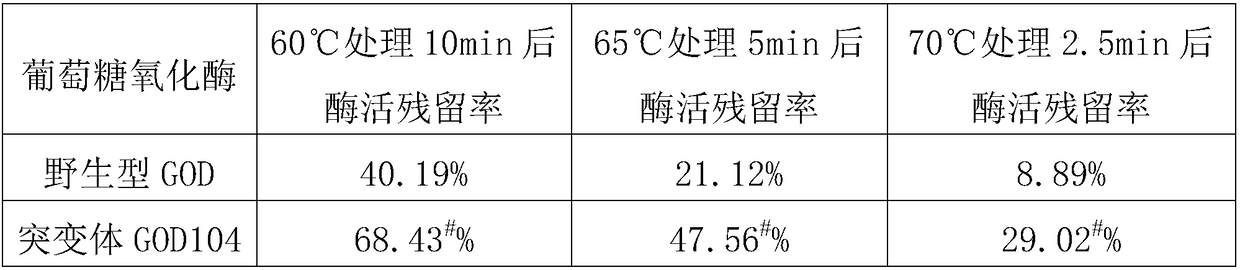
The invention aims to provide a heat-resistant glucose oxidase mutant. Remained enzyme activity is 29.02% and remarkably higher than that of a wild type after the glucose oxidase mutant containing V104I single-site mutation is processed for 2.5 minutes at the temperature of 70 DEG C. In order to further improve heat resistance of a glucose oxidase three-point mutant GOD-K (A65R, H275F and A416K),V104I single-site mutation and combination of mutation sites of V104I and T32L and / or A290L are introduced, and results display that the heat resistance of an acquired mutant is remarkably higher thanthat of the GOD-K, and the remaining enzymatic activity of the mutant reaches up to 83.10% after acquired mutant is processed for 2.5 minutes at the temperature of 75 DEG C and is improved by 46.42%as compared with that of the GOD-K, so that the mutant is more suitable for the field of feed processing.
Owner:QINGDAO VLAND BIOTECH GRP
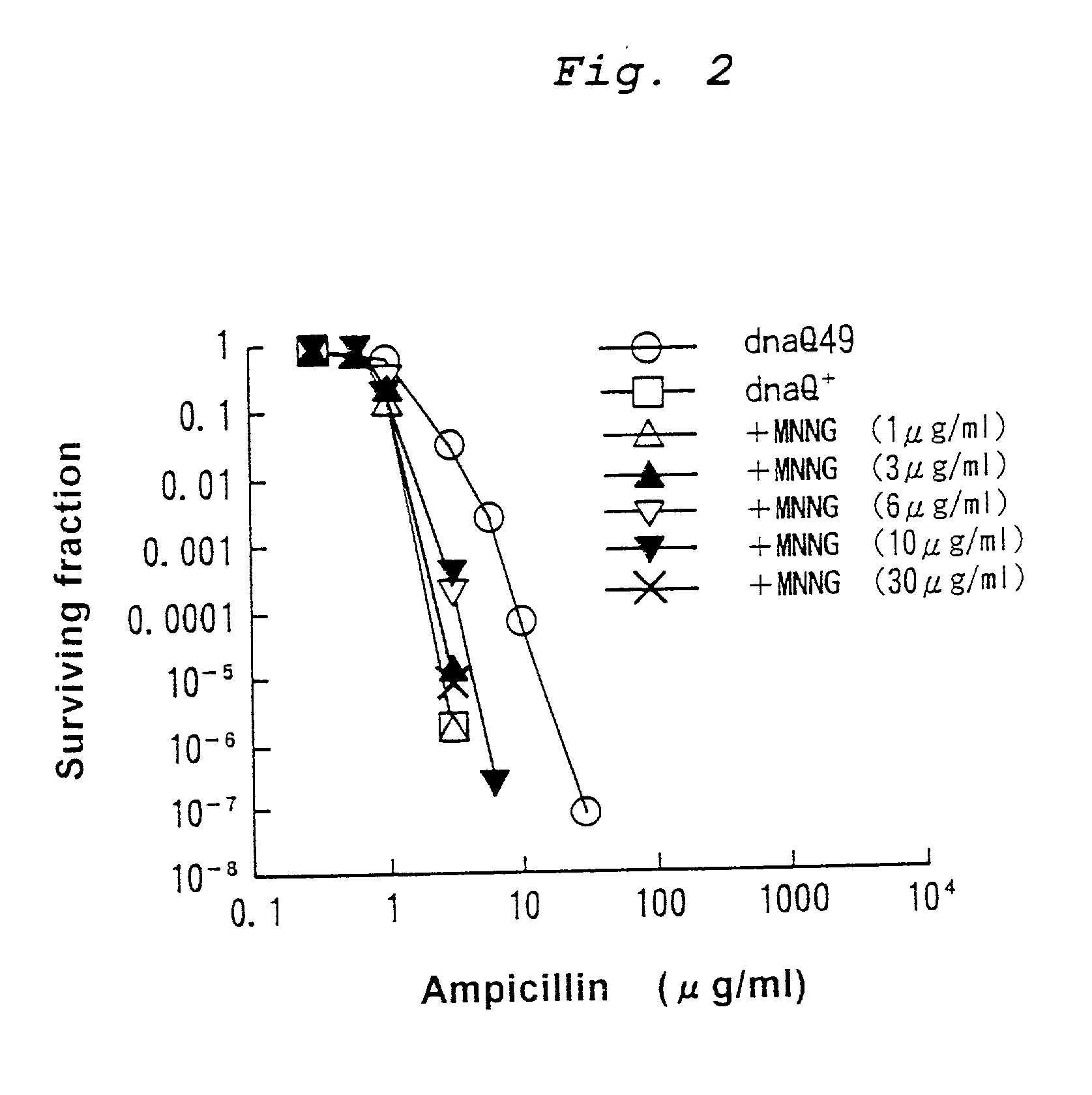
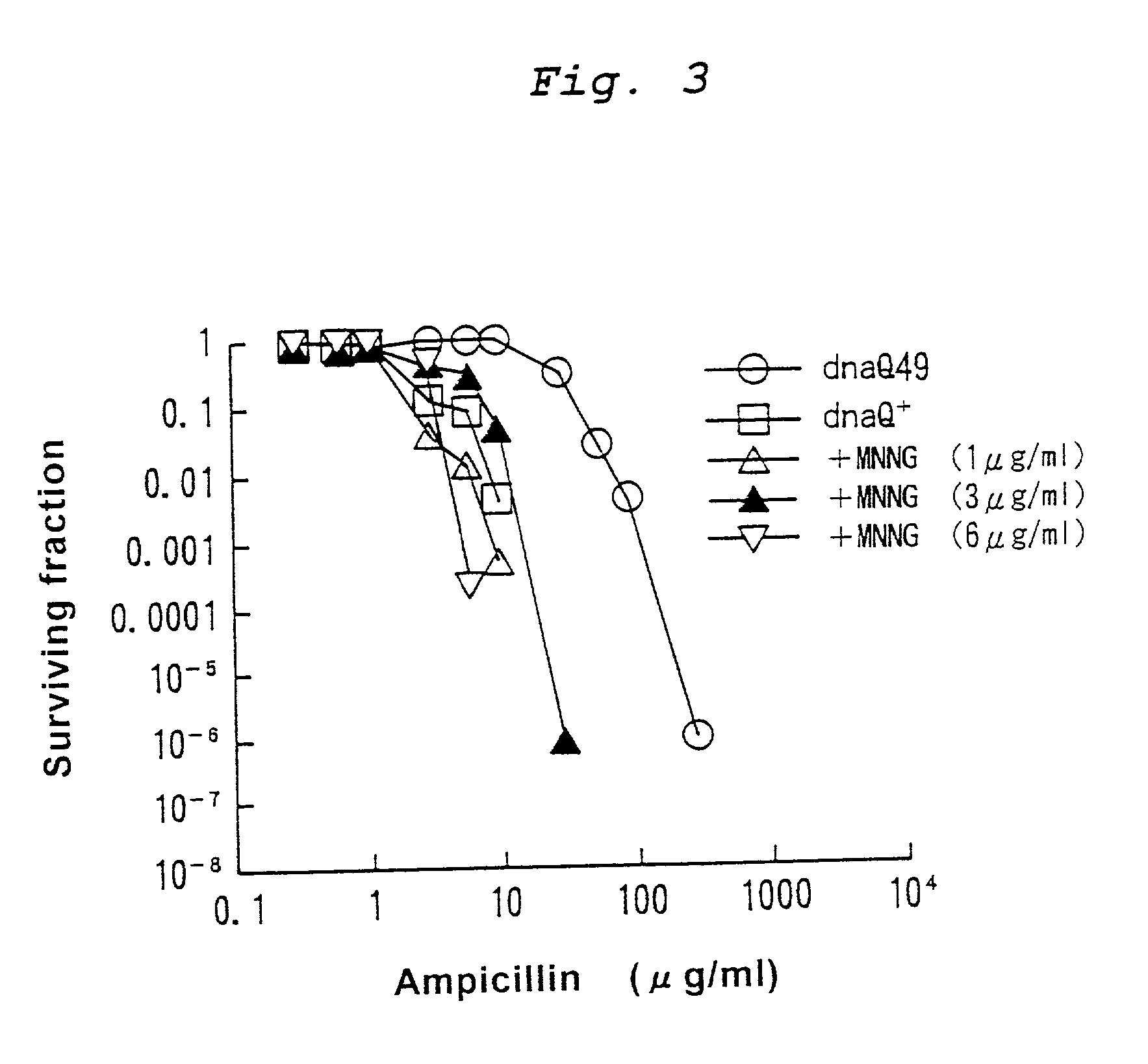
Mutagenesis method
InactiveUS20030124725A1Efficient and effective introductionReducing risk of extinctionBacteriaMutant preparationGenomic DNACancer metastasis
This application provides a method for mutagenesis of a gene, which comprises introducing much more point mutations into one strand of double-stranded genomic DNA of cell or organism individual than into another strand. In accordance with such a method, it is now possible to efficiently and effectively construct various useful mutants of microorganisms, cells or organism individuals. It is also now possible by analyzing the mutating conditions of the gene to clarify the mechanism of drug resistance, to estimate the occurrence of a novel insensible microorganism or to develop a drug therefor, to analyze the mutation of an oncogene and the mechanisms of cancer metastasis and increase in malignancy, to develop a therapeutic method using these mechanisms, etc.
Owner:JAPAN SCI & TECH CORP
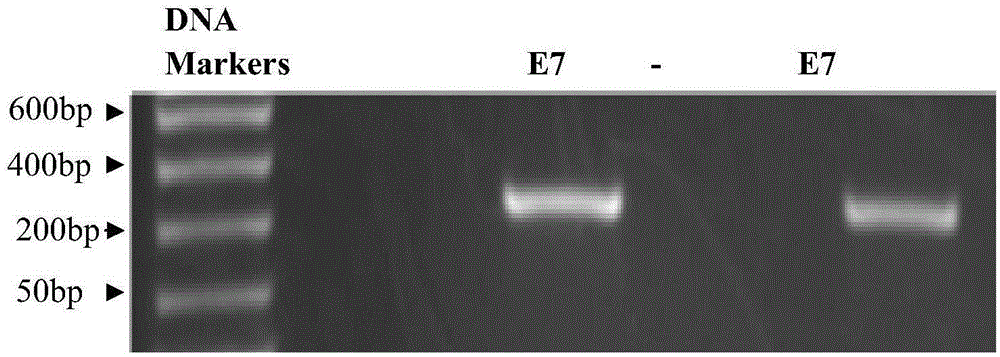
Recombinant adeno-associated viral vector with human papillomavirus type 16 multi-point mutant E7<mm> antigen genes, method for constructing recombinant adeno-associated viral vector and application thereof
The invention discloses a recombinant adeno-associated viral vector with HPV-16 (human papillomavirus-16) E7<mm> antigen genes and a method for constructing the recombinant adeno-associated viral vector. The method includes mutating tumorigenicity HPV-16 E7 antigen genes at multiple points to obtain non-tumorigenicity E7 antigen genes; inserting the mutated genes into an adeno-associated viral vector without structural genes to obtain the recombinant adeno-associated viral vector. The recombinant adeno-associated viral vector and the method have the advantages that the mutant HPV-16 E7<mm> antigen genes carried by recombinant adeno-associated viruses can be delivered into monocyte-dendritic cell lines to be used for stimulating effect cells of immune systems; as proved by experiments, growth of HPV-16 E7 positive cells can be effectively inhibited by CTL (cytotoxic lymphocytes) induced by DC (dendritic cells) infected by the recombinant adeno-associated viruses for patients in in-vitro and in-vivo manners, or the HPV-16 E7 positive cells can be effectively killed by the CTL for the patients in the in-vitro and in-vivo manner, and tumorigenicity can be prevented; the recombinant adeno-associated viral vector or associated products can be used for preparing anti-tumor medicine for treating HPV-16 infection or diseases associated with the HPV-16 infection.
Owner:GUANGDONG TOPHEALTH BIOTECH
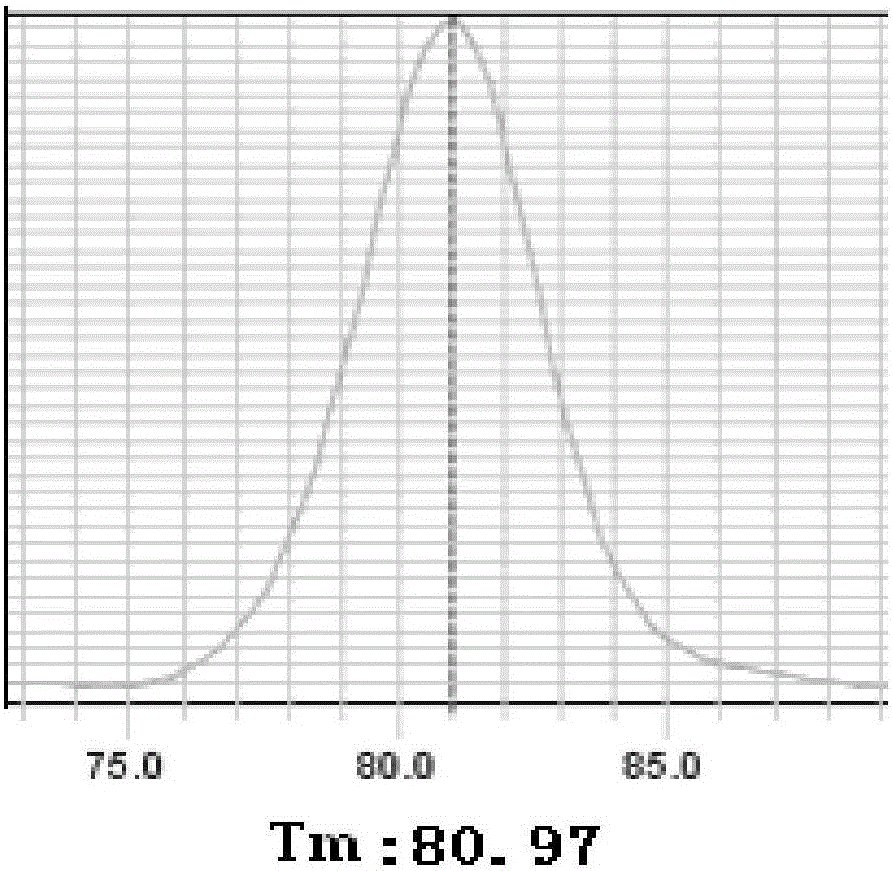
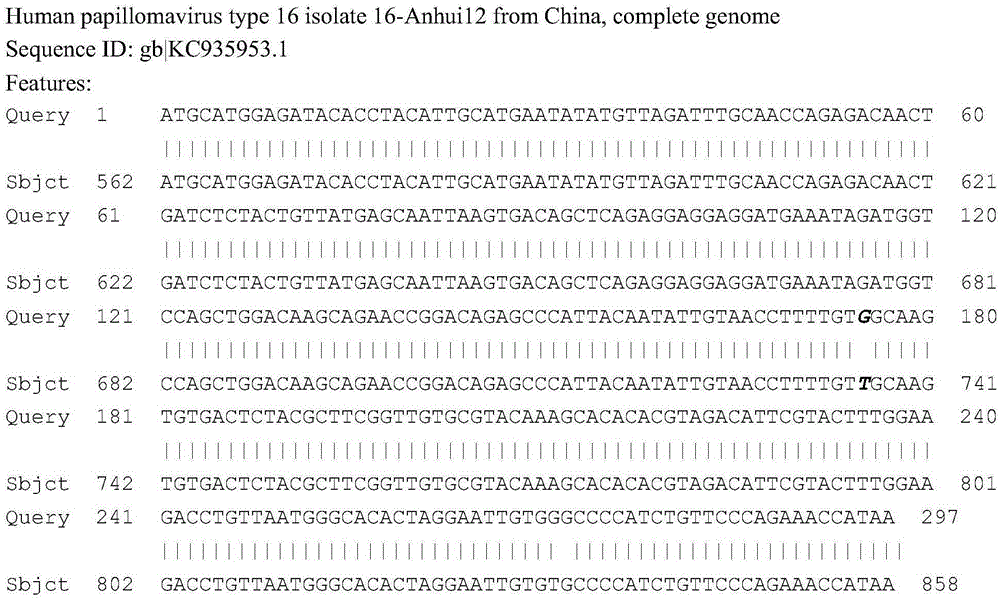
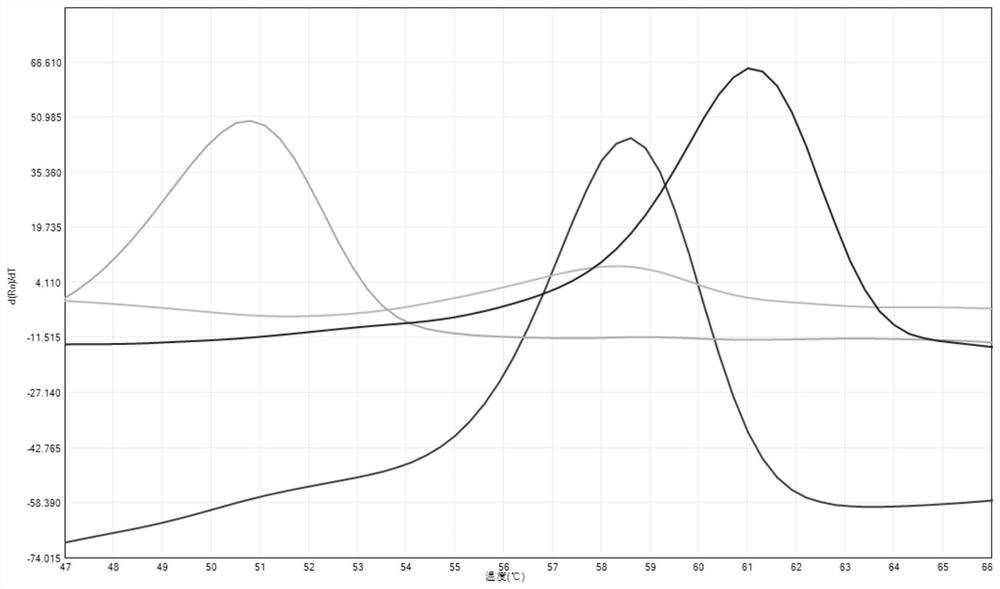
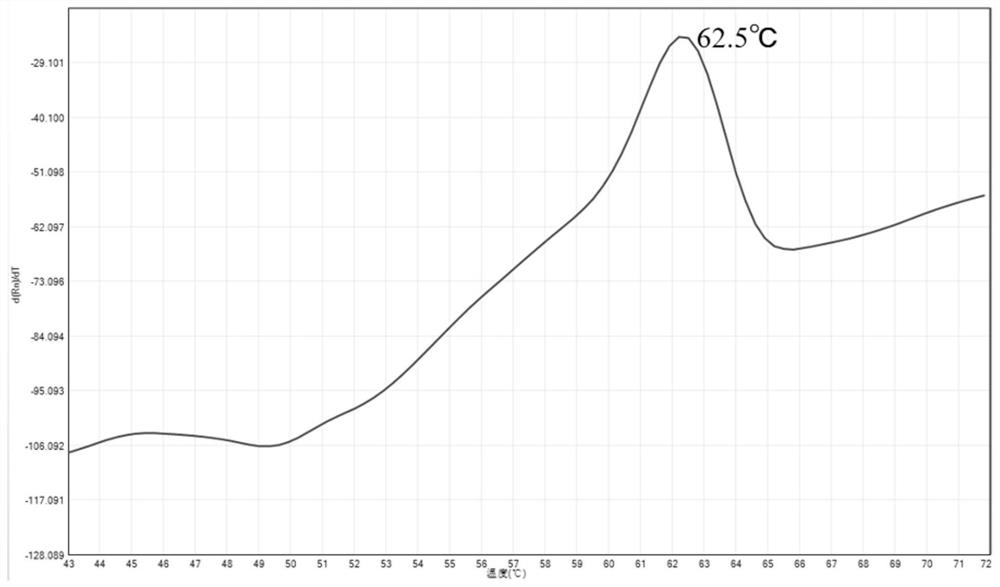
Fluorescent PCR detection method for simultaneously detecting deletion mutation and point mutation of genes by single tube
PendingCN111909990AImprove featuresGood repeatabilityMicrobiological testing/measurementFluorescent pcrDeletion mutation
The invention relates to the technical field of gene detection, in particular to a fluorescent PCR detection method for simultaneously detecting deletion mutation and point mutation of genes by a single tube. The method comprises the following steps: screening out available primers and probes, wherein the Tm value of the primers is higher than that of the probes; performing PCR reaction in the same reaction system of the single tube by using the obtained primers and probes; detecting change of the fluorescence value of the PCR reaction by using an instrument, analyzing and acquiring a channelfluorescence signal corresponding to the designed probe, and judging whether a detected sample has certain deletion mutation or not according to existence of melting peaks of all fluorescence channels; judging whether the detected sample has certain point mutation or not according to the temperatures of the melting peaks of all the fluorescence channels. The method has the beneficial effects thata plurality of deletion mutations and a plurality of point mutations can be simultaneously detected by only adding genome DNA of the detected sample into a detection reagent, one-time closed tube operation is realized, and pollution of a PCR product to an experimental environment is eliminated.
Owner:亚能生物技术(深圳)有限公司
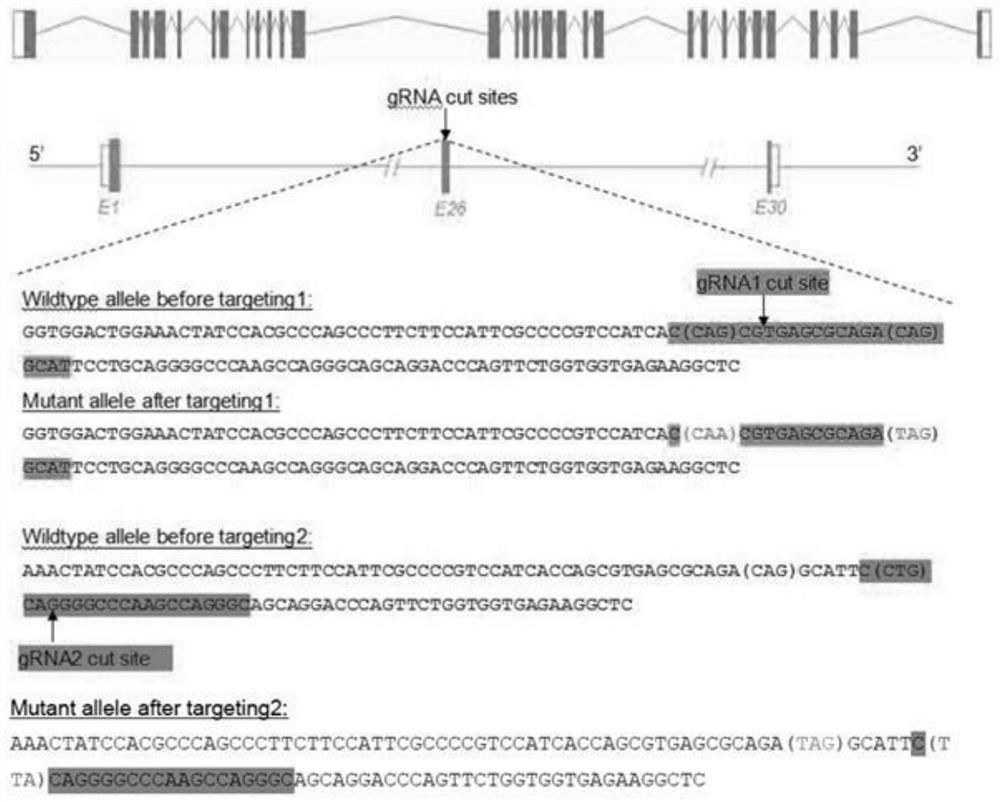
Construction method of point mutation glanzmann's thrombasthenia (GT) mouse model
ActiveCN112063654APrecision editingNucleic acid vectorVector-based foreign material introductionWild typeSexual maturity
The invention provides a construction method of a point mutation glanzmann's thrombasthenia (GT) mouse model. The construction method comprises the following steps of: (1), performing carrier design and construction, and in-vitro transcription: (1-1), designing sequences of gRNA and Donor Oligo according to gene information and experimental requirements; (1-2), synthesizing the Donor Oligo, and constructing a gRNA carrier; and (1-3), performing in-vitro transcription on the gRNA vector and a Cas9 vector; (2), performing microinjection and identification of F0-generation mice; (3), breeding andidentifying F1-generation mice: respectively breeding the sexually mature positive F0 mice in the step (2) with wild mice for one generation, and finally, providing at least three F1-generation pointmutation GT mouse models verified by PCR (Polymerase Chain Reaction) and sequencing; and (4), freezing and preserving breeds. A CRISPR / Cas9 technology is used for accurately editing and constructingthe point mutation GT mouse model for the first time; the mouse gene is accurately edited through the model; compared with a GT mouse model constructed through antibody injection induction or a gene knockout technology in the past, the model is more accurate; and a good model is provided for deeply researching the pathogenesis of the GT and exploring a new treatment method.
Owner:AFFILIATED HOSPITAL OF NANTONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com