Cyclodextrin glycosyl transferase with improved maltodextrin substrate specificity and preparation method thereof
A technology of substrate specificity and glycosyltransferase, applied in botany equipment and methods, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems of maltodextrin substrate specificity (low conversion rate, etc.) , to achieve the effect of improving substrate specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Cyclodextrin Glucosyltransferase with Improved Substrate Specificity
[0024] The cyclodextrin glycosyltransferase of the present invention is obtained on the basis of the gene sequence published by GenBank AF047363.1 by performing single or compound mutations on the amino acids at four positions, and specifically obtained 15 mutants, namely K47L, Y89F, N94P, D196Y, K47L / Y89F, K47L / N94P, K47L / D196Y, Y89F / N94P, Y89F / D196Y, N94P / D196Y, K47L / Y89F / N94P, K47L / Y89F / D196Y, K47L / N94P / D196Y, Y N94P / D196Y, K47L / Y89F / N94P / D196Y.
[0025] Amino acid substitutions can be carried out at the four sites in the mature region by chemical total synthesis or site-directed mutagenesis.
Embodiment 2
[0026] Example 2: Preparation method of cyclodextrin glucosyltransferase with improved substrate specificity
[0027] This example uses the PCR method as an example for illustration, but the protection of the invention is not limited to the method of obtaining mutations only through the PCR method. Mutant enzymes K47L, Y89F, N94P, D196Y, K47L / Y89F, K47L / N94P, K47L / D196Y, Y89F / N94P, Y89F / D196Y, N94P / D196Y, K47L / Y89F / N94P, K47L / Y89F / D196Y, K47L / N94 / D196Y, Y89F / N94P / D196Y, K47L / Y89F / N94P / D196Y are prepared as follows:
[0028] 1) Site-directed mutation
[0029] Site-directed mutagenesis of single mutant enzymes K47L, Y89F, N94P and D196Y, using the rapid mutation kit MutanBEST kit to express vector cgt / pET-20b(+) 1 for the template,
[0030] The primers for site-directed mutagenesis introducing the K47L codon are:
[0031] Forward primer: 5′-CCAATTTG CTG CTCTATTTCGGGGG-3′, the underline is the mutant base;
[0032] Reverse primer: 5′-ATCGGTCGCCGCTGAACGCA-3′;
[0033] The...
Embodiment 3
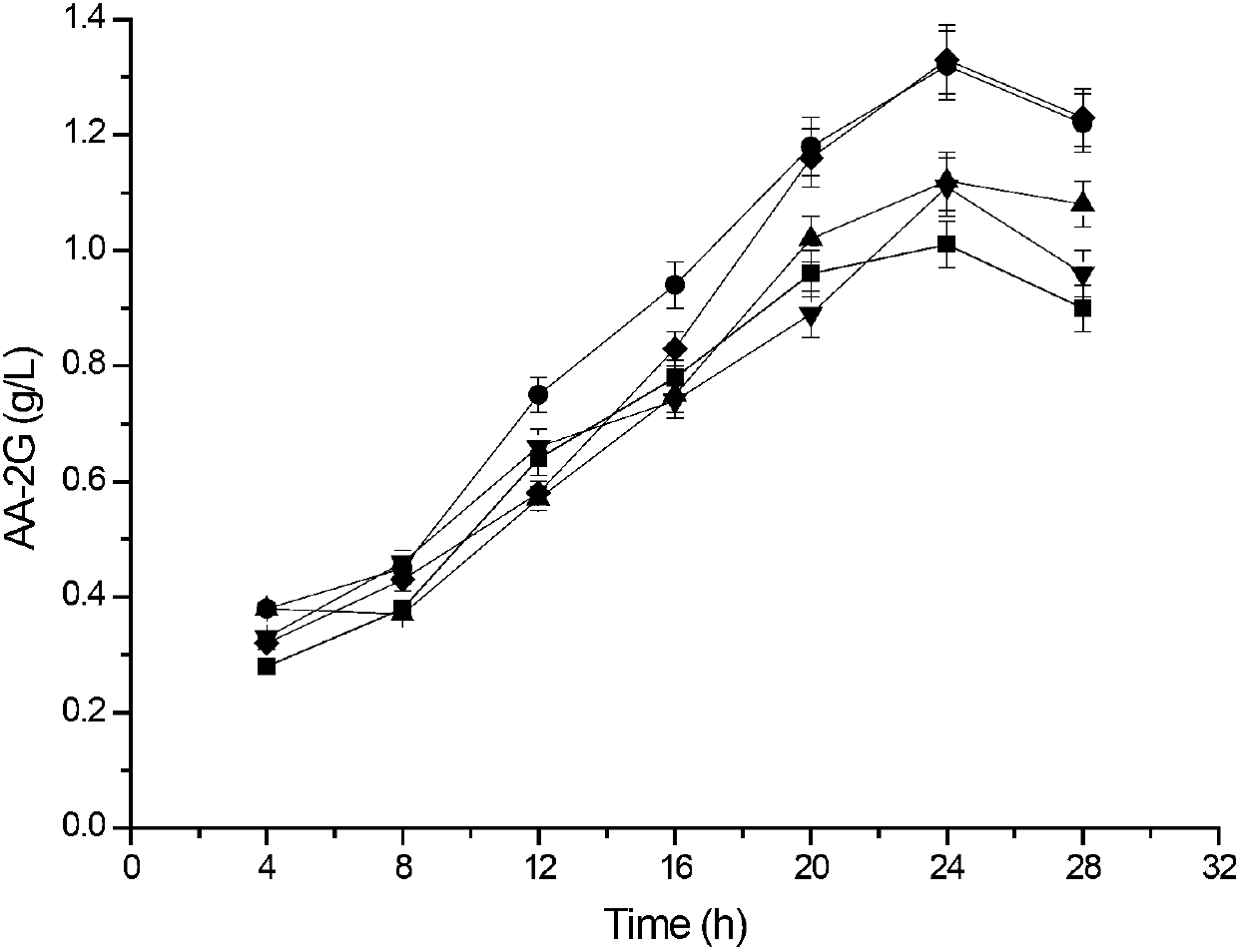
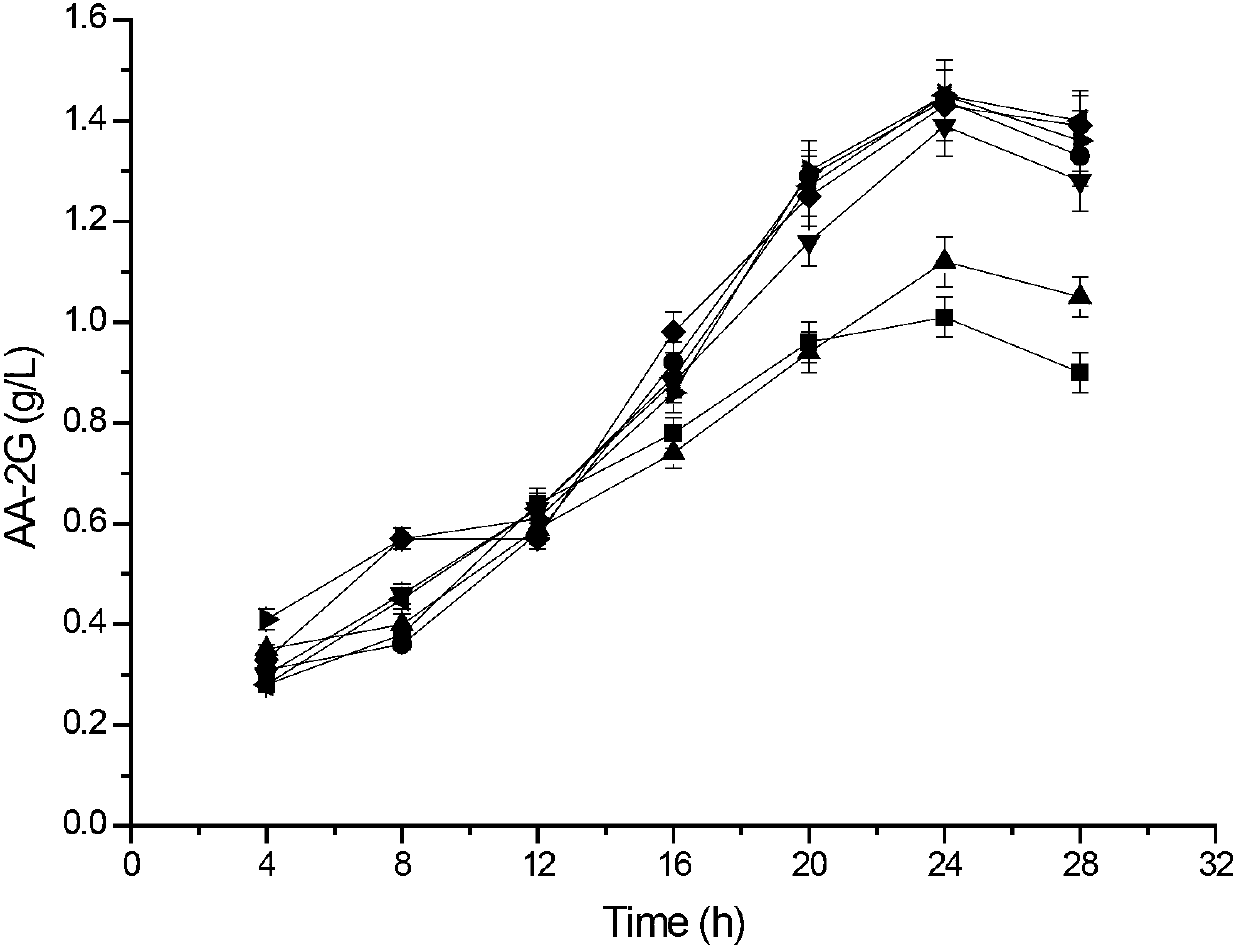
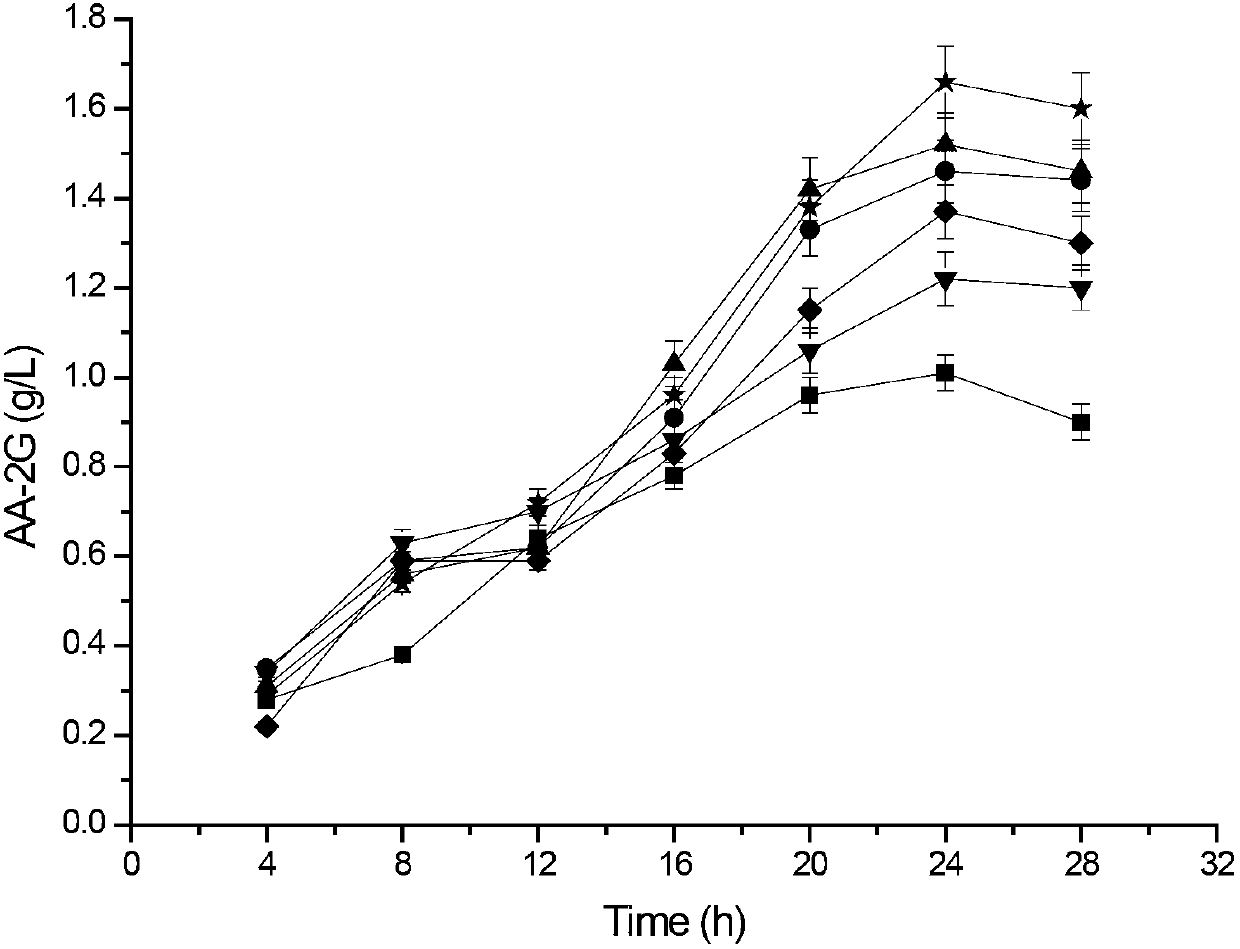
[0087] Example 3: This example illustrates the analysis of enzyme activity and the synthesis and detection of AA-2G.
[0088] 1) Enzyme activity assay method:
[0089] Method for measuring α-cyclization activity by methyl orange method: Take 0.1mL of appropriately diluted enzyme solution, add it to 0.9mL of 3% soluble starch solution prepared in advance with 50mM phosphate buffer (pH6.5), at 40°C After reacting for 10 min, add 1.0 mL of 1.0 M hydrochloric acid to stop the reaction, then add 1.0 mL of 0.1 mM methyl orange prepared with 50 mM phosphate buffer, incubate at 16°C for 20 min, and measure the absorbance at 505 nm. One unit of enzyme activity defines the amount of enzyme required to generate 1 μmol α-cyclodextrin per minute under this condition.
[0090] Determination of starch hydrolysis activity: add an appropriate amount of enzyme solution to 50mM phosphate buffer (pH 6.5) containing 2% soluble starch, react at 50°C for 10min, and then use the DNS method to measur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com